Abstract
Background: Type 2 diabetes mellitus (T2DM) is increasingly prevalent and associated with increased risk for cardiovascular and renal disease. Therefore, right selection of antidiabetic medications is one of the important tools for managing cardiovascular disease in people with diabetes. American Diabetes Association (ADA) recommends use of combination therapy for better management of T2DM. Objective: The purpose of this study was to elucidate the effectiveness of glimepiride and metformin in long-duration T2DM, with or without other oral hypoglycemic agents (OHAs), in the Indian setting. Material and methods: A retrospective, multicenter, observational, case-based questionnaire survey was conducted at multiple health care facilities in India using the medical records of T2DM patients who were prescribed various glimepiride/metformin strengths. Results: This retrospective observational questionnaire-based analysis comprised 5,097 T2DM patients in total. The mean (±SD) age of patients having diabetes for ≤10 years and >10 years are 51.6 ± 11.54 and 59.75 ± 12.47 years, with BMI 28.47 ± 4.64, and 29.35 ± 5.05 kg/m2, respectively. The majority (66.54%) of patients came from the less affluent category. Combination of glimepiride/metformin was given to about 4,143 patients in various doses as first-line therapy, and 954 additional patients received the combination in various doses as second-line therapy. About 2.8% of the patients (p = 0.001) complained of hypoglycemia.
It was observed that combination therapy of glimepiride and metformin was significantly effective in achieving glycemic control among patients with T2DM for long duration. The study also showed that the drug combination was found to be effective in 95.4% (p = 0.001) patients and well-tolerated in 95.05% (p = 0.001) patients, hence the combination was rated by physicians as good to excellent. Conclusion: This study demonstrates the effectiveness of glimepiride and metformin, both individually and in combination with other OHAs. In Indian patients with T2DM, the combination of glimepiride and metformin resulted in satisfactory glycemic control and was well-tolerated.
Keywords: Type 2 diabetes mellitus, ADA, glimepiride/metformin
Introduction
Diabetes is a chronic condition resulting from either inadequate insulin production by the pancreas or impaired insulin utilization by the body. While insulin resistance can arise from various factors, obesity and aging are among the most common contributors1.
Type 2 diabetes mellitus (T2DM) is an urgent global threat to the health and well-being of individuals, families, and society as its incidence is on rise globally2. According to the International Diabetes Federation (IDF)
Diabetes Atlas, 10th edition, 1 in 10 adults, or 537 million people, have diabetes. It is expected that this figure would increase to 643 million by 2030 and 783 million by 2045. Approximately 75% of adults with diabetes live in underdeveloped or developing countries.
In 2021, diabetes was responsible for 6.7 million deaths3.
The scenario is even more adverse in India, as it continues to take the form of an epidemic leading to disability and fatal complications that are related to an increased financial burden4.
Long-term diabetes increases the risk of various microvascular and macrovascular diseases in patients. Poor glycemic control further elevates the likelihood of developing macrovascular complications, such as coronary artery disease, peripheral arterial disease, and stroke or transient ischemic attack. Additionally, it raises the risk of microvascular complications, including diabetic nephropathy, neuropathy, and retinopathy2.
First-line treatment options for diabetes typically include monotherapy, with metformin being a common choice. Other antidiabetic drugs include insulin secretagogues, sulfonylurea (SU) (glimepiride), insulin sensitizers, alpha-glucosidase inhibitors, incretin mimetics, amylin antagonists, and sodium-glucose co-transporter-2 (SGLT2) inhibitors are the main medications used to treat type 2 diabetes. Patients who are unable to accomplish treatment goals with first-line oral hypoglycemic agents (OHAs) are recommended combination therapy5.
According to the UK Prospective Diabetes Study (UKPDS), glycemic control using a single OHA is likely to be ineffective in the longer duration of disease, thus, unavoidably; many patients will require combination therapy to achieve their target glucose level6.
Several other global bodies like American Diabetes Association/European Association, International Diabetes Federation (IDF), South Asian Federation of Endocrine Societies (SAFES), and Research Society for the Study of Diabetes in India (RSSDI), World Health Organization (WHO) have recommended the use of modern SUs like glimepiride in T2DM management singly or in combination7-10.
The combination of SU (glimepiride) and metformin is frequently considered for treating T2DM due to its ability to treat "insulin secretion disorder" and "insulin resistance", respectively. Numerous strengths of the fixed-dose combination (FDC) of glimepiride and metformin are offered and frequently utilized by general practitioners and specialists in India11.
The current study is aimed to analyze the usage and efficiency of the glimepiride and metformin combination in the management of long duration T2DM.
Material and Methods
Study Design
This was a retrospective, multicenter, observational, case-based questionnaire survey. It was conducted with 534 health care professionals (HCPs) across different centers in India from November 2009 to July 2022.
Study Population
Patients of sexes, aged above 18 years, diagnosed with T2DM who received glimepiride (0.5/1/2/3/4 mg)/metformin (500/850/1000 mg) in different strengths were recruited in the study. About 4,143 patients (3,878 patients with diabetes duration ≤10 years and 265 patients with diabetes duration >10 years) received different strengths of glimepiride/metformin as first-line therapy and 954 patients (741 patients with diabetes duration ≤10 years and 213 patients with diabetes duration >10 years) received different strengths of glimepiride/metformin as second-line therapy (Table 1).
Data Collection
A case report format was developed to determine the pattern of use of different strengths of glimepiride/ metformin FDCs with or without other OHAs in diabetes patients. The questionnaire was sent to 534 HCPs across India via an online portal. Questions regarding demographic characteristics, such as age, sex, body mass index (BMI), weight change, and economic class; duration of diabetes; antidiabetic drugs used
|
Table 1. Patient Demographics and Treatment
|
| |
Diabetes duration
|
P value
|
|
≤10
|
>10
|
|
Patient age
|
51.6 ± 11.54
|
59.75 ± 12.47
|
<0.001
|
|
Patient BMI
|
28.47 ± 4.64
|
29.35 ± 5.05
|
<0.001
|
|
Glimepiride/Metformin first-line therapy
|
3,878 (93.6)
|
265 (6.4)
|
<0.001
|
|
Glimepiride/Metformin second-line therapy
|
741 (77.67)
|
213 (22.33)
|
|
(glimepiride/metformin); antidiabetic drug up-titrations and down-titrations; weight change; hypoglycemic episodes, follow-up and adherence to lifestyle, were included in the questionnaire. An online portal was developed where the HCPs filled in the information. A descriptive analysis was performed with the data provided on the portal.
Statistical Analysis
All continuous variables are expressed as mean ± SD or median with the interquartile range per the data distribution. Categorical variables are expressed as numbers and their respective percentage. Differences in binary and ordinal variables between two independent groups were analyzed by the exact Chi-square test. All the reported p-values are two-sided, and p-values <0.05 are considered to indicate statistical significance.
All data entries and statistical analyses were performed by using SPSS@ Version 23.0 software.
Results
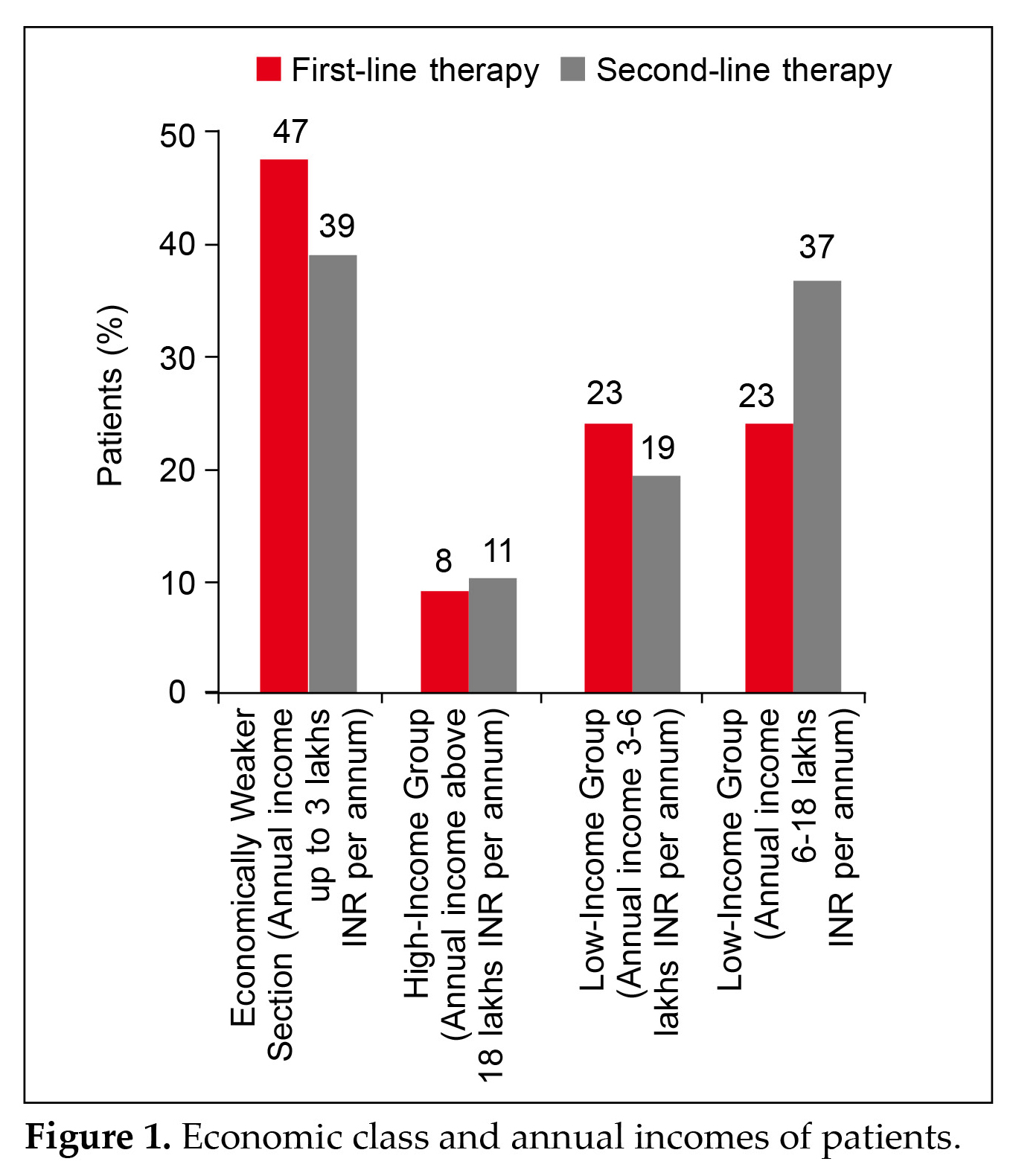
A total of 5,097 patients with T2DM were included in this retrospective observational questionnaire-based analysis. The mean (±SD) age of patients having diabetes ≤10 years and >10 years are 51.6 ± 11.54 and 59.75 ± 12.47 years, respectively. The mean (±SD) BMI of patients having diabetes ≤10 years and >10 years are 28.47 ± 4.64 and 29.35 ± 5.05 kg/m2, respectively. Most of the patients belonged to the less affluent category (66.54%). Figure 1 summarizes the economic class and annual incomes of patients.
Four thousand three hundred thirty-four patients (85.03%) found the drug combination available all the time (100%), and 649 (12.73%) found it available 75% of the time. The rest of the patient population found it available <25% of the time.
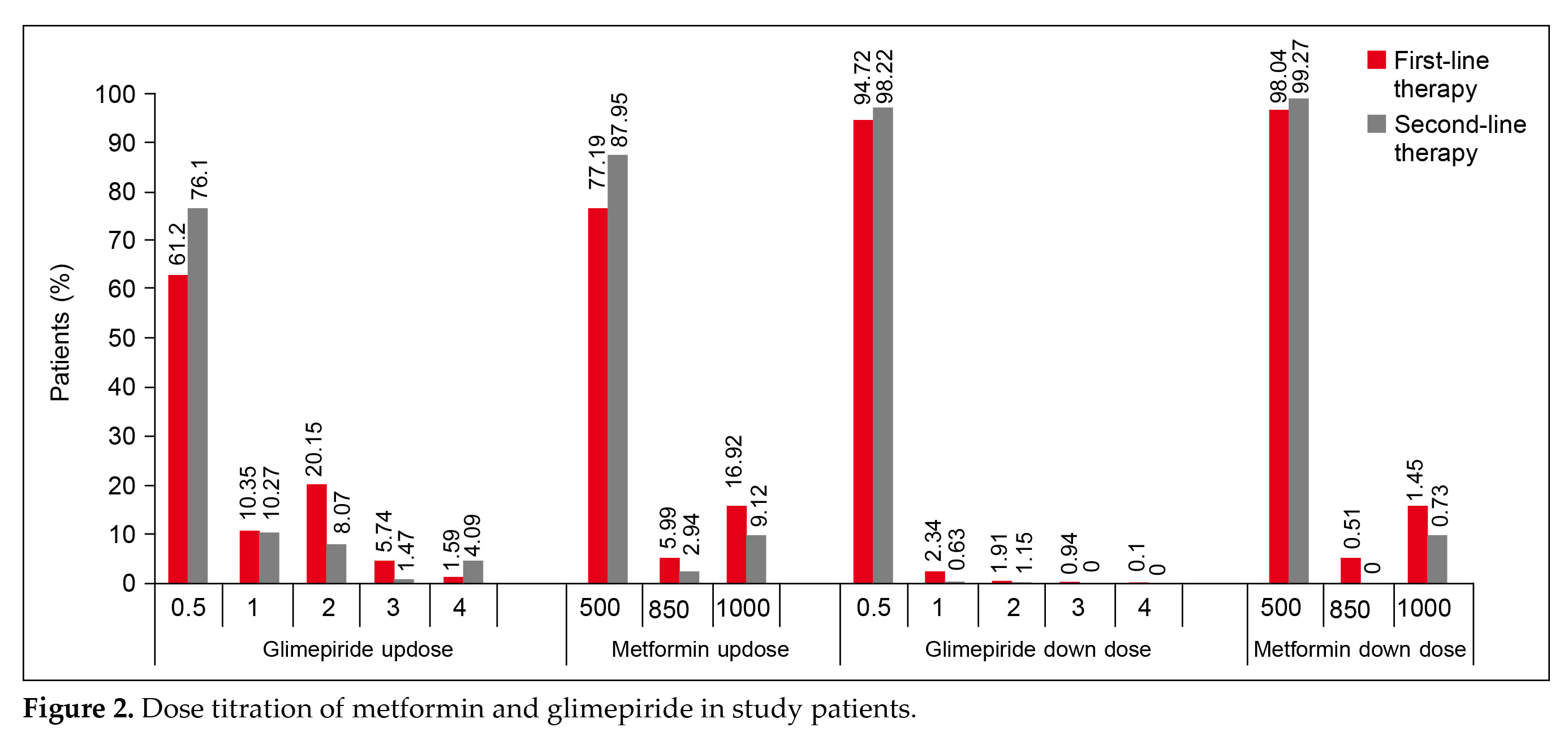
Dose titration was prevalent in patients receiving glimepiride/metformin as first-line therapy (Fig. 2).
Up-titration was done in 1,678 patients (40.5%) while down-titration was done in 285 patients (6.88%).
Up-titration and down-titration in patients receiving other OHAs along with glimepiride/metformin were 310 (32.49%) and 30 (3.14%), respectively.
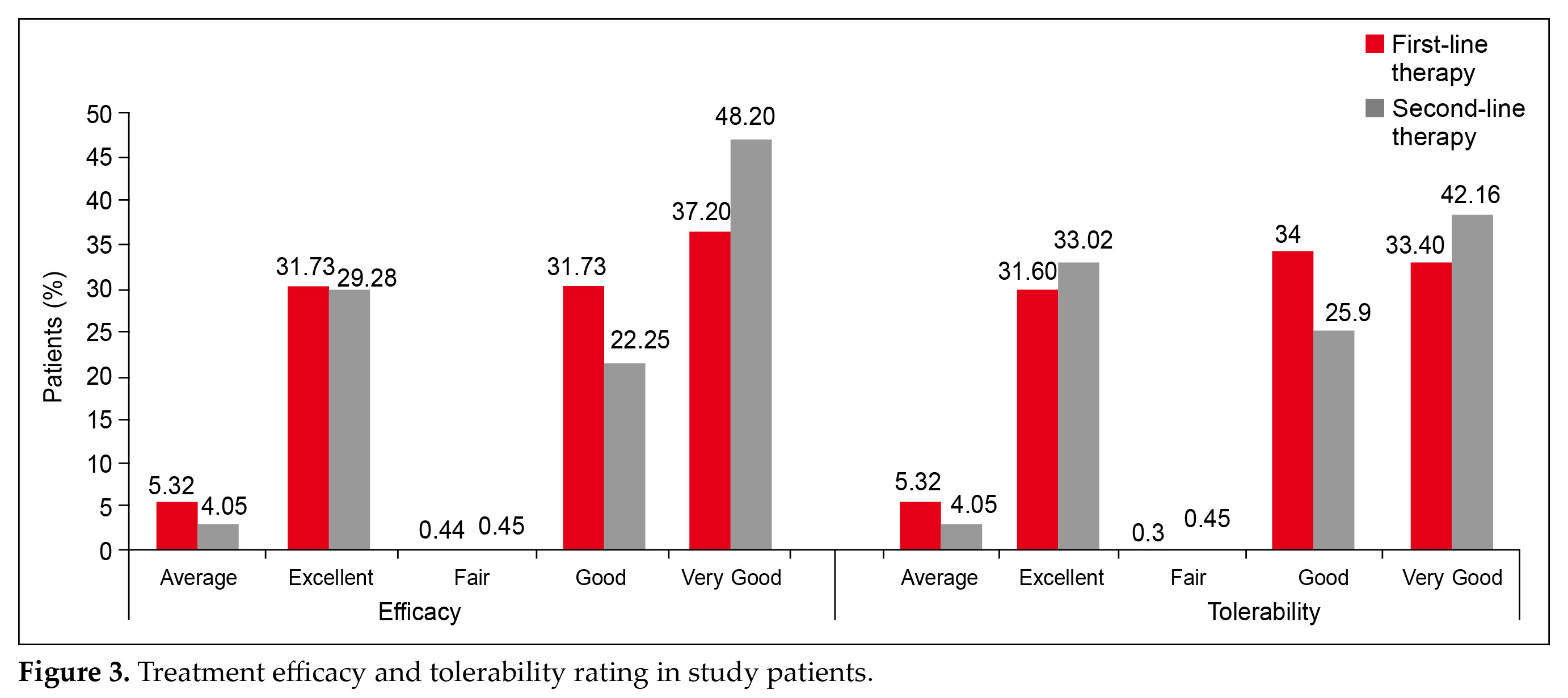
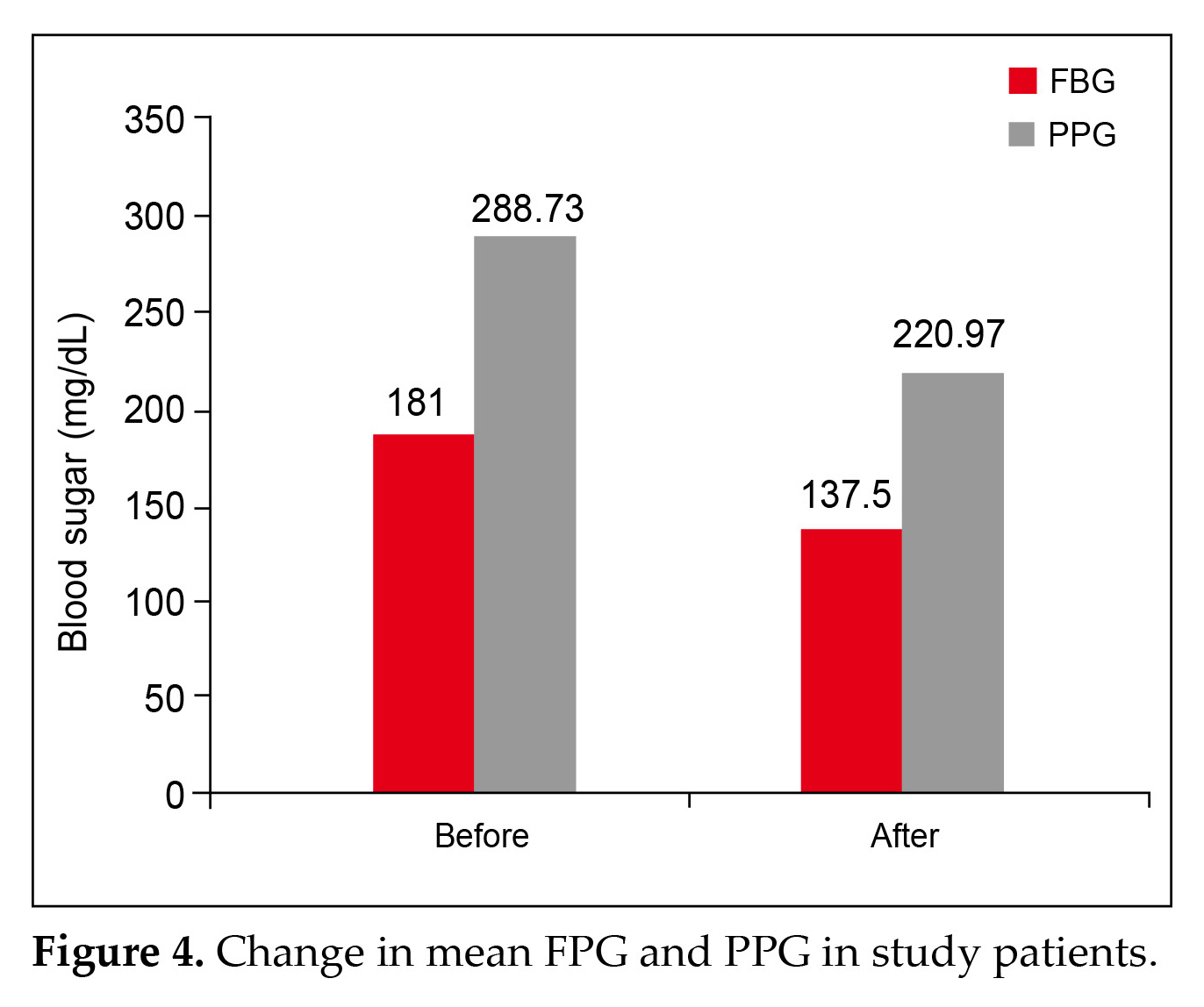
Physician evaluation of efficacy and tolerability were reported as good to excellent for 95.4% (p < 0.001) and 95.05% (p < 0.001) patients, respectively (Fig. 3). The main efficacy outcomes were changes in fasting blood glucose (FBG), postprandial plasma glucose (PPG), glycated hemoglobin (HbA1c), and body weight. FBG decreased from 181 mg/dL to 137.5 mg/dL
on an average, while PPG lowered from 288.73 mg/dL to 220.97 mg/dL (Fig 4).
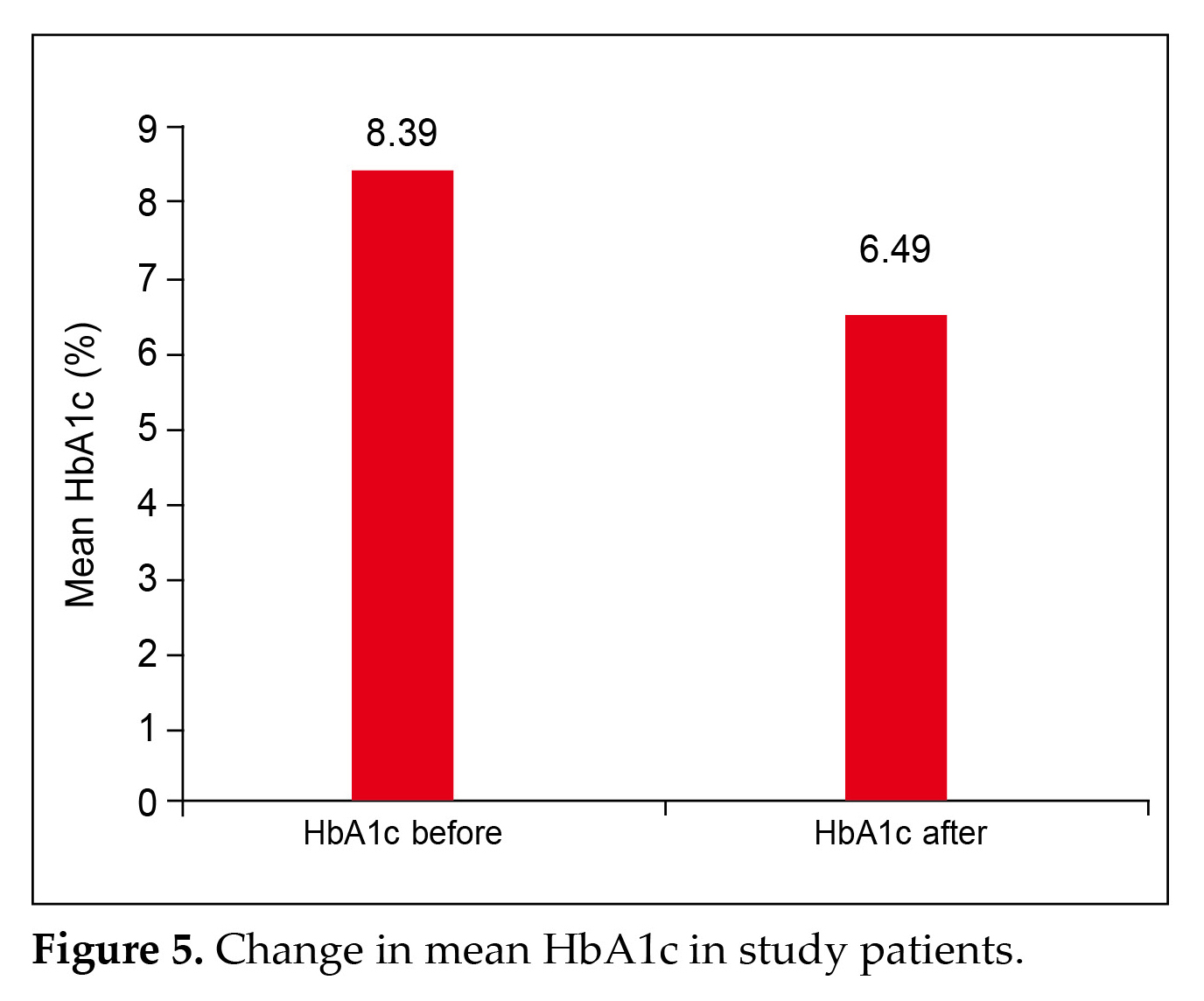
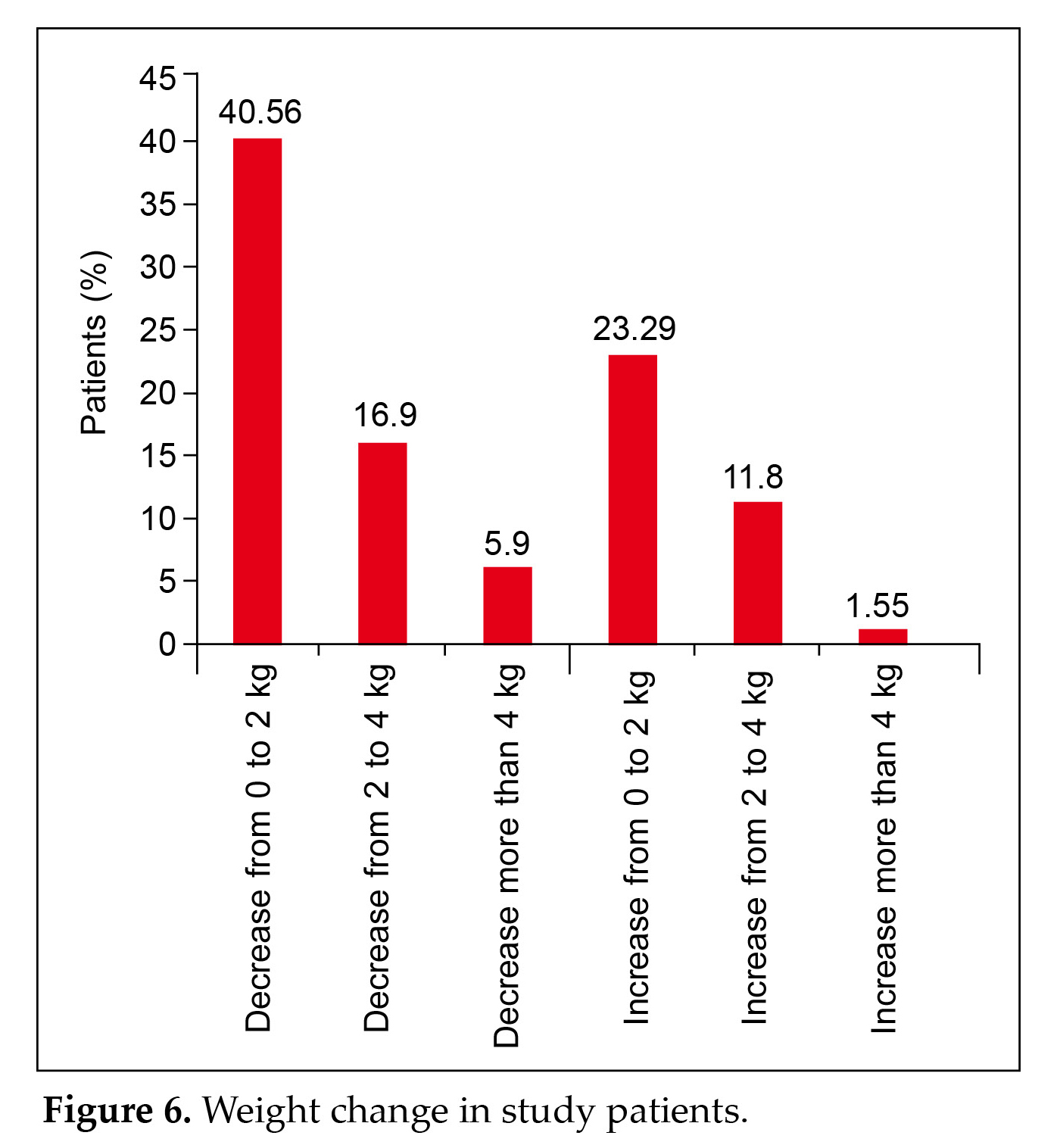
A significant (p = 0.0423) reduction in the HbA1c value from 8.39% to 6.489% was observed (Fig 5). Weight loss was evident in most patients (63.36%) (Fig. 6).
In this case-based questionnaire survey, out of 5,097 patients, only 154 patients experienced a hypoglycemic event (in the last 6 months) (p < 0.002). It was observed that 87.93% patients in the first-line therapy and 63% patients in the second-line therapy adhered to follow ups with doctors as per the advice given. Additionally, the majority of the patients (89.89%) in the first-line therapy and 89.1% patients in the second-line therapy adhered to a healthy lifestyle.
Discussion
Metformin increases insulin sensitivity, while glimepiride increases beta-cell glucose sensitivity and promotes endogenous insulin production. A comple-mentary mechanism of action between glimepiride and metformin results in a considerable decrease in glycemic indices (FPG, PPG, and HbA1c levels). When compared to older generation SUs, Glimepiride a newer SU offers a number of benefits, including enhanced beta-cell activity, weight-neutral effects, absence of cardiovascular risk, and reduced hypoglycemia episodes12-16. This study analyzed the use of a glimepiride and metformin combination in Indian patients with
long-standing T2DM. It included two groups of long-term diabetic patients: those with duration of diabetes <10 years, and those with duration of diabetes of ≥10 years.
Detailed effects of the drug combination on different parameters such as FBG, PPG, HbA1c, and body weight are included. It further highlights the physicians’ opinion regarding the efficacy and tolerability of the combination drug.
A study by Nybäck-Nakell et al (2014) demonstrated that SUs may lose their effectiveness over time due to beta-cell exhaustion. However, research has explored the durability of glycemic control achieved through the combination of SUs and metformin in patients with diabetes for both <10 years and ≥10 years17.
On similar lines, the current study, showed the mean (±SD) age of patients having diabetes ≤10 years and >10 years to be 51.6 ± 11.54 and 59.75 ± 12.47 years, respectively. Four thousand six hundred nineteen patients (90.62%), with a mean age of 51.6 ± 11.54 years, and 478 patients (9.37%), with a mean age of 59.75 ± 12.47 received glimepiride/metformin.
The current results indicate good glycemic control in both the above mentioned categories and showed a sustained legacy effect maintained for a longer duration of diabetes, which was in concordance with the study conducted by Kalra et al11. In this study, 4,334 patients (85.03%) found the drug combination available all the time (100%), and 649 (12.73%) found it available 75% of the time. The rest of the patient population found it available <25% of the time. Additionally, the major patient population availing the drug combination belonged to an economically weaker section. Similar observation have been made in different studies which imply that glimepiride/metformin are superior or at least
noninferior to other expensive medications, have been used for longer periods of time, and are available in cheap generic formulae which would benefit the huge patient population in India who mostly belong to the less affluent category18-21.
Dose titration was prevalent in patients receiving glimepiride/metformin as first-line therapy (Fig. 2). Up-titration was done in 1,678 patients (40.5%) while down-titration was done in 285 patients (6.88%). Up-titration and down-titration in patients receiving other OHAs along with glimepiride/metformin were 310 (32.49%) and 30 (3.14%), respectively.
A current case-based questionnaire conducted in Indian patients shows that the glimepiride and metformin combinations are beneficial to T2DM patients irrespective of age, diabetes duration, BMI, and comorbidities from diabetes. These combinations are advantageous, to physicians especially in areas with limited resources due to the simplicity of up- and down-titrations22.
Physician evaluation of efficacy and tolerability were reported as good to excellent for 95.4% (p < 0.001) and
95.05% (p < 0.001) patients, respectively (Fig. 3). This is in accordance with the studies shown in a recent retrospective, nonrandomized, noncomparative, multicentric real-world study all the strengths of the glimepiride and metformin combinations are widely prescribed in diabetes with comorbidities like hypertension and dyslipidemia and complications for optimal glycemic control. Glimepiride and metformin FDCs are suitable for early as well as long-term diabetes4.
Several studies have shown that the addition of glimepiride in T2DM patients uncontrolled by metformin alone resulted in superior glycemic control than metformin monotherapy. Additionally the combined use of glimepiride-metformin in a single presentation was efficacious and safe in patients with T2DM23-25.
In the current study, a significant decrease in the FBG, PPG, and HbA1c was observed, which is in similar lines with the findings by Phung et al (2010), Hassan and Abd-Allah (2015), Kumar (2021), and Shrivastava et al (2023)13,26-28.
Our findings found that weight loss was evident in most patients (63.36%) (Fig. 6). Drug combinations containing the modern SU glimepiride + metformin exhibit synergistic activity in terms of glycemic management and superior safety profile by lowering hypoglycemia, weight gain, and cardiovascular profile in comparison to previous generation SUs. Weight loss contributes to improved glycemic control in many obese individuals with diabetes. Hassanein et al (2020) reported comparable weight loss outcomes in the DIA-RAMADAN trial13,29,30.
This study also reported that only 2.8% of patients (p < 0.001) experienced hypoglycemia. The combination of glimepiride/metformin achieves good glycemic control and tolerability. In a recent study, Prasanna Kumar et al also reported a similar finding that stated the efficacy and tolerability to be good to excellent (97.3% and 96.6%) in a vast majority of patients31. In another international prospective study, treatment with glimepiride showed fewer hypoglycemic episodes compared to those treated with glibenclamide among patients with diabetes32.
Glimepiride's documented cardiovascular safety/neutrality and reduced hypoglycemia episodes make it an attractive alternative for the management of persons with long-standing diabetes33.
Modern SUs offer superior glycemic efficacy, have better cardiovascular profile and are also available at a reasonable cost. Treatment with modern SUs is associated with a lower economic burden, and hence they are an effective alternative to other newer antidiabetic drugs11.
Treatment decisions are based on glycemic effectiveness and safety profiles, as well as the influence of the therapy on weight and hypoglycemia risk, comorbidities, and treatment costs. The combination of glimepiride and metformin is a viable choice for managing long-term T2DM in developing countries like India.
Conclusion
In conclusion, the study demonstrates that the combi-nation of the SU glimepiride and metformin is a common treatment choice for T2DM. This FDC offers multiple benefits and is widely used by general practitioners and specialists across India.
The current study focuses on evaluating the use and effectiveness of the glimepiride and metformin combination in managing long-term T2DM, providing insights into its role in this patient population. The combination of glimepiride and metformin provided effective glycemic control and was well-tolerated.
Major Findings
- Glimepiride (0.5/1/2/3/4 mg) and metformin (500/850/1000 mg) combinations are widely available in clinical practice.
- In young (<60 years) as well as elderly populations (>60 years) with long-term diabetes (>5 years), glimepiride and metformin combinations are efficacious and well-tolerated.
- The main efficacy outcomes were changes in HbA1c, FBG, PPG, and body weight. There was a significant (p = 0.0423) reduction in the HbA1c value. Weight loss was evident in the majority of patients (63.36%).
Acknowledgment
We thank Dr Siddhi Shetty and Ms Prarthana Sinha (USV team members) for their support in conducting the study.
References
- Sapra A, Bhandari P. Diabetes. [Updated 2023 Jun 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551501/
- American Diabetes Association Professional Practice Committee. Summary of Revisions: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(Suppl 1):S5-S10.
- IDF Diabetes Atlas; Diabetes around the world in 2021: IDF Diabetes Atlas. 10th Edition and other resources, ADA; 2023 [cited 2023 9-Jun-23]. Available from: https://diabetesatlas.org.
- Sahay RK, Mittal V, Gopal GR, Kota S, Goyal G, Abhyankar M, et al. Glimepiride and metformin combinations in diabetes comorbidities and complications: real-world evidence. Cureus. 2020;12(9):e10700.
- Padhi S, Nayak AK, Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharmacother. 2020;131:110708.
- Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab. 2016;20(4):546-51.
- Kalra S, Aamir AH, Raza A, Das AK, Azad Khan AK, Shrestha D, et al. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: a consensus statement. Indian J Endocrinol Metab. 2015;19(5):577-96.
- Aschner P. New IDF clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes Res Clin Pract. 2017;132:169-70.
- Bajaj S. RSSDI clinical practice recommendations for the management of type 2 diabetes mellitus 2017. Int J Diabetes Dev Ctries. 2018;38(Suppl 1):1-115.
- Guidelines on second- and third-line medicines and type of insulin for the control of blood glucose levels in non-pregnant adults with diabetes mellitus. Geneva: World Health Organization; 2018. Available from: https://www.who.int/publications/i/item/9789241550284. Accessed April 15, 2025.
- Kalra S, Das AK, Baruah MP, Unnikrishnan AG, Dasgupta A, Shah P, et al. Glucocrinology of modern sulfonylureas: clinical evidence and practice-based opinion from an International Expert Group. Diabetes Ther. 2019;10(5):1577-93.
- Basit A, Riaz M, Fawwad A. Glimepiride: evidence-based facts, trends, and observations (GIFTS). [corrected]. Vasc Health Risk Manag. 2012;8:463-72.
- Shrivastava A, Kesavadev J, Mohan V, Saboo B, Shrestha D,Maheshwari A, et al. Clinical evidence and practice-based guidelines on the utility of basal insulin combined oral therapy (metformin and glimepiride) in the current era. Curr Diabetes Rev. 2023;19(8):e090123212444.
- Jindal S, Ganopadhyay KK, Santhosh R, Donagaon S, Chopra V, Paserkar G et al. Efficacy of the nodern SU glimepiride in reducing hyperglycemia in T2DM. J Assoc Physicians India. 2020;67: p. 33.
- Zhou JB, Bai L, Wang Y, Yang JK. The benefits and risks of DPP4-inhibitors vs. sulfonylureas for patients with type 2 diabetes: accumulated evidence from randomised controlled trial. Int J Clin Pract. 2016;70(2):132-41.
- Riddle MC. A verdict for glimepiride: effective and not guilty of cardiovascular harm. Diabetes Care. 2019;42(12):2161-3.
- Nybäck-Nakell Å, Adamson U, Lins PE, Landstedt-Hallin L. Adding glimepiride to insulin+metformin in type 2 diabetes of more than 10 years' duration -- a randomised, double-blind, placebo-controlled, cross-over study. Diabetes Res Clin Pract. 2014;103(2):286-91.
- Zhang Y, McCoy RG, Mason JE, Smith SA, Shah ND, Denton BT. Second-line agents for glycemic control for type 2 diabetes: are newer agents better? Diabetes Care. 2014;37(5):1338-45.
- Gu S, Tang Z, Shi L, Sawhney M, Hu H, Dong H. Cost-minimization analysis of metformin and acarbose in treatment of type 2 diabetes. Value Health Reg Issues. 2015;6:84-8.
- Singh SK. Commentary on “Consensus recommendations on sulfonylurea and sulfonylurea combinations in the management of type 2 diabetes mellitus: International Task Force”. Indian J Endocrinol Metab. 2018;22(1):158-9.
- Tandon T, Dubey AK, Srivastava S, Manocha S, Arora E, Hasan N. A pharmacoeconomic analysis to compare cost-effectiveness of metformin plus teneligliptin with metformin plus glimepiride in patients of type-2 diabetes mellitus. J Family Med Prim Care. 2019;8(3):955-9.
- Unnikrishnan AG, Pandit K, George J, Venkataraman S, Abhyankar MV. Clinical utilization pattern of multiple strengths of glimepiride and metformin fixed dose combinations in Indian type 2 diabetes patients. J Assoc Physicians India. 2020;68(7):57-61.
- Kalra S, Bahendeka S, Sahay R, Ghosh S, Md F, Orabi A, et al. Consensus recommendations on sulfonylurea and sulfonylurea combinations in the management of type 2
diabetes mellitus - International Task Force. Indian J Endocrinol Metab. 2018;22(1):132-57.
- Charpentier G, Fleury F, Kabir M, Vaur L, Halimi S. Improved glycaemic control by addition of glimepiride to metformin monotherapy in type 2 diabetic patients. Diabet Med. 2001;18(10):828-34.
- González-Ortiz M, Martínez-Abundis E; Grupo para el Tratamiento de la Diabetes Mellitus con Combinaciones. Eficacia y seguridad de la terapia hipoglucemiante oral combinada de glimepirida más metformina en una sola forma farmacéutica en pacientes con diabetes mellitus tipo 2 y falla secundaria a monoterapia con glibenclamida [Efficacy and safety of glimepiride plus metformin in a single presentation, as combined therapy, in patients with type 2 diabetes mellitus and secondary failure to glibenclamide, as monotherapy]. Rev Invest Clin. 2004;56(3):327-33.
- Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303(14):1410-8.
- Hassan MH, Abd-Allah GM. Effects of metformin plus gliclazide versus metformin plus glimepiride on cardiovascular risk factors in patients with type 2 diabetes mellitus. Pak J Pharm Sci. 2015;28(5):1723-30.
- Kumar S. Comparison of safety and efficacy of glimepiride-metformin and vildagliptin-metformin treatment in newly diagnosed type 2 diabetic patients.Indian J Endocrinol Metab. 2021;25(4):326-31.
- Das AK, Saboo B, Chawla R, Aravind SR, Rajput R, Singh AK, et al. Time to reposition sulfonylureas in type 2
diabetes management in Indian context: a pragmatic practical approach. Int J Diabetes Dev Ctries. 2023:1-19.
- Hassanein M, Al Sifri S, Shaikh S, Abbas Raza S, Akram J,
Pranoto A, et al; DIA-RAMADAN Study Investigators. A real-world study in patients with type 2 diabetes mellitus treated with gliclazide modified-release during fasting: DIA-RAMADAN. Diabetes Res Clin Pract. 2020;163:108154.
- Prasanna Kumar KM, Seshadri K, Aravind SR, Deb P, Modi KD, Gopal RA, et al. Real-world observational study of glimepiride and metformin fixed-dose combination along with insulin in the management of type 2 diabetes mellitus: Indian experience. Cureus. 2021;13(1):e13020.
- Zekry R, Omran GA, El-Gharbawy NM, Werida RH. Comparative study of dapagliflozin versus glimepiride effect on insulin regulated aminopeptidase (IRAP) and interleukin-34 (IL-34) in patient with type 2 diabetes mellitus. Sci Rep. 2023;13(1):6302.
- ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al; American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2023. Diabetes Care. 2022;46(Suppl 1):S140-57.