Published in IJCP
April-June 2025
Review Article
Mitochondrial Dysfunction in Type 2 Diabetes Mellitus: Imeglimin as a Novel Therapeutic Approach
June 30, 2025 | Surender Kumar, Rishi Shukla, Shambo Samajdar, Soumik Goswami, Om Lakhani, Prem Kumar, Sameer Muchhala, Niddhi Baxi
Diabetes & Endocrinology
Abstract
Insulin resistance (IR) in peripheral tissues, such as skeletal muscle and adipose tissue, is recognized as a precursor to type 2 diabetes mellitus (T2DM). Mitochondria play a vital role in the pathogenesis of T2DM, primarily attributed to the oxidative stress and systemic inflammation caused by the compromised mitochondrial function, which further results in IR. Imeglimin, a mitochondrial modulator, has been shown to enhance insulin secretion, achieve optimal glucose control, and improve insulin sensitivity. The available evidence suggests that imeglimin can boost mitochondrial bioenergetics, regulate respiratory chain functions, and reduce reactive oxygen species (ROS) production, while protecting endothelial cells without affecting mitochondrial respiration. In T2DM patients, imeglimin improves beta-cell glucose responsiveness and stimulates insulin secretion via multiple pathways. Imeglimin has been shown to be well-tolerated and effective in the management of T2DM, both as a standalone treatment and in combination with other oral therapies, with evidence indicating improvements in insulin sensitivity and reductions in blood glucose levels. All these insights point towards imeglimin being a unique drug in the management of T2DM, making it an important addition to the armamentarium of diabetes treatment.
Keywords: Beta-cells, imeglimin, insulin secretion, mitochondrial dysfunction
Introduction
Optimal glycemic control in type 2 diabetes mellitus (T2DM) remains a pressing global concern, as underscored by the 2025 International Diabetes Federation (IDF) Diabetes Atlas, which estimates that approximately 11.1% of the global adult population (20-79 years), equivalent to 1 in 9 individuals is living with diabetes, with over 40% of cases remaining undiagnosed.. Projections from the IDF Diabetes Atlas (2025) indicate that by 2050, approximately 1 in 8 adults, an estimated 853 million individuals, will be living with diabetes, representing a 46% increase from current figures1,2. In India, the overall prevalence of diabetes, based on data from 15 states, is 7.3%. Managing diabetes poses multiple challenges, including substantial health care costs, suboptimal patient adherence, and limitations in drug utilization due to contraindications and side effects associated with available therapeutic classes. This scenario underscores the ongoing need for a medication that comprehensively addresses the multifaceted pathogenesis of T2DM.
Insulin resistance (IR) in the peripheral tissues like skeletal muscles and adipose tissues has been identified as a precursor to T2DM. This IR frequently builds up leading to hyperglycemia and overstimulation of pancreatic beta-cells. When the pancreatic beta-cells are overstimulated, they compensate by elevating the insulin secretion. However, prolonged overstimulation of these cells leads to functional impairment and loss, finally resulting in T2DM4.
Mitochondria play a vital role in the pathogenesis of T2DM, primarily attributed to the oxidative stress and systemic inflammation caused by the compromised mitochondrial function, which further results in IR5. The role of mitochondria in the pathogenesis of metabolic diseases like obesity, metabolic syndrome, and T2DM is the focus of many ongoing research studies. From the aforementioned literature, it is reasonable to conclude that T2DM results from the inability of the insulin-sensitive tissues to respond to insulin as well as insufficient insulin secretion by pancreatic beta-cells, both contributing to hyperglycemia. Hence, there is a need for a drug that could effectively manage both conditions at the same time6.
Imeglimin can be a suitable choice for this dual problem. Imeglimin, a tetrahydrotriazine compound chemically known as (6R)-(+)-4-dimethylamino-2-imino-6-methyl-1,2,5,6-tetrahydro-1,3,5-triazine hydrochloride, targets mitochondrial bioenergetics, and in addition to this, enhances glucose-stimulated insulin secretion (GSIS), preserves beta-cell mass, improves insulin sensitivity, reduces hepatic glucose output, and strengthens insulin signaling in the liver and skeletal muscles. It is a unique standalone drug with multiple effects that include augmentation of GSIS, maintenance of beta-cell mass, improvement of insulin action, diminution of the hepatic glucose output, and enhancement of insulin-signaling pathway in both the liver and skeletal muscles. At the molecular level, imeglimin rectifies the mitochondrial dysfunction by correcting the respiratory chain activity with partial suppression of Complex I and improvement of Complex III activity, reducing reactive oxygen species (ROS) formation (thus preventing the mitochondrial permeability transition pore [mPTP] opening, which otherwise could lead to cell death) and improving ATP generation7.
Imeglimin was initially authorized for use in Japan and China in 2021, and it became available in India starting October 20228. It has been found to be well-tolerated and an effective treatment option for lowering A1c, both as monotherapy and in combination with other antihyperglycemic agents, in phase 2 and phase 3 trials.
Imeglimin can help manage diabetic complications such as metabolic cardiomyopathy, diabetic vasculopathy, and diabetic neuroinflammation. Multiple pivotal phase 3 trials of imeglimin have demonstrated statistically significant glucose-lowering properties and a favorable safety and tolerability profile9. This article delves into the role of imeglimin in modulating the mitochondrial pathogenesis of T2DM.
Role of Mitochondria in the Pathogenesis of T2DM
Mitochondria and Oxidative Phosphorylation
While glucose is the primary determinant of insulin secretion, other metabolites such as free fatty acids, long-chain acyl-coenzyme A, and glutamate also enhance insulin secretion. Pancreatic beta-cells have a unique metabolic pathway where most of the pyruvate derived from glucose enters mitochondria to undergo the tricarboxylic acid (TCA) cycle. Mitochondrial oxidative phosphorylation (OXPHOS) and subsequent ATP production play a crucial role in triggering insulin secretion. Decreased expression of OXPHOS genes has been observed in beta cells from patients with
T2DM10-13.
Excessive Nutrition and Sedentary Lifestyle
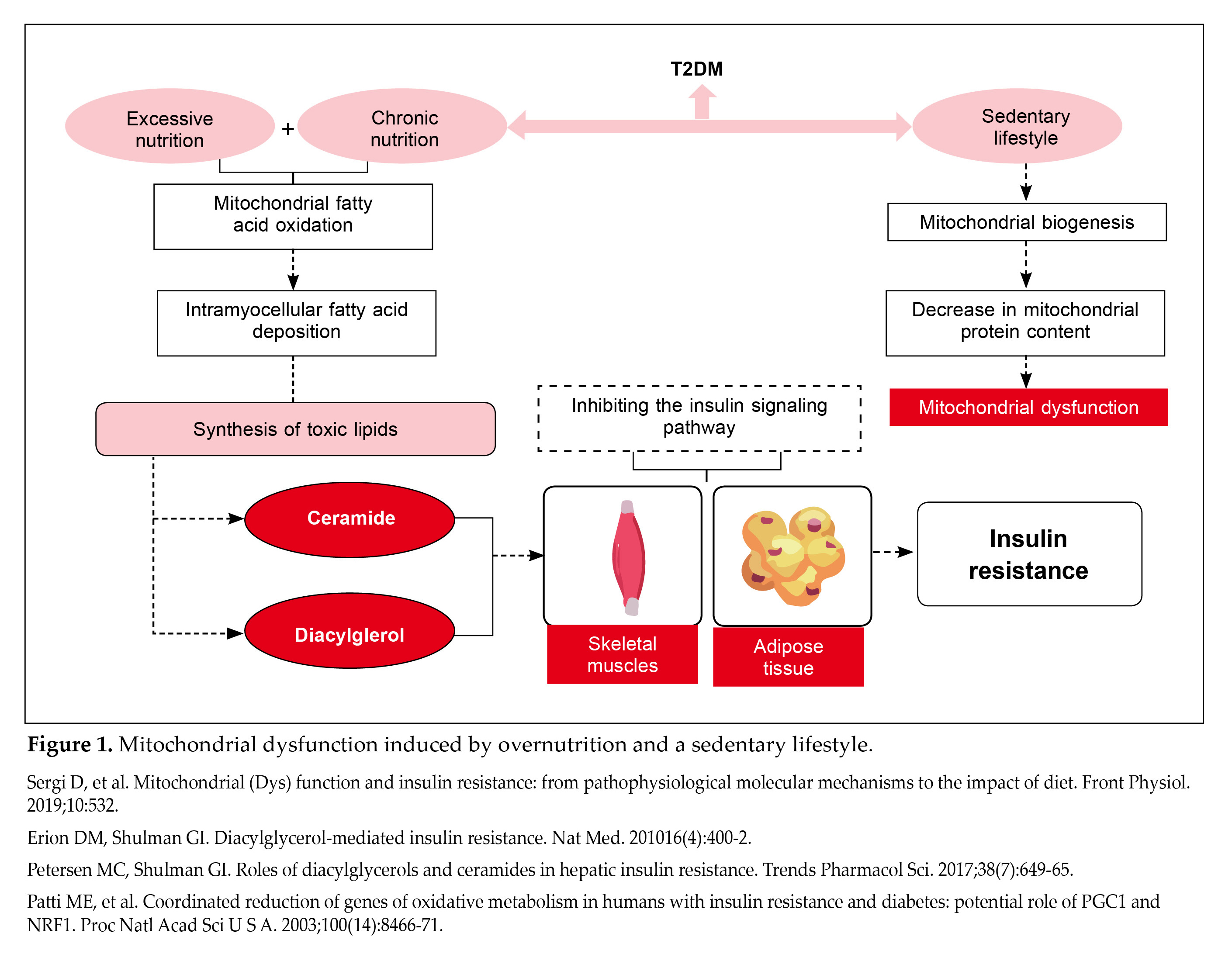
T2DM is intricately tied to overconsumption of food and a sedentary lifestyle, resulting in a surplus of calories. This excess of nutrients causes mitochondria to increase their oxidation of fatty acids as a compensatory response. However, excessive nutrition for prolonged duration exceeds the capacity of mitochondrial fatty acid oxidation, leading to the accumulation of fatty acids within muscle cells. These fatty acids may then be altered into harmful lipids such as ceramide and diacylglycerol. The presence of ceramide and diacylglycerol can act as dual threats as they inhibit insulin signaling pathways in skeletal muscles and adipose tissues, contributing to IR (Fig. 1)14,15.
Another aspect to ponder at is the physical inactivity in patients with diabetes. A sedentary lifestyle associated with T2DM leads to a decrease in mitochondrial protein content, predominantly because of reduced mitochondrial biogenesis, which contributes to mitochondrial dysfunction16.
However, mitochondrial dysfunction may also be secondary to IR. Studies have shown that insulin-resistant cells exhibit reduced mitochondrial energy production and increased susceptibility to oxidative stress17. Therefore, mitochondrial dysfunction plays a significant role in promoting IR through various mechanisms. The debate continues as to whether mitochondrial dysfunction is a primary contributor to IR or a consequence of it, but its role in exacerbating IR is well-established. Enhancing mitochondrial function is thus an important and validated approach to increase insulin sensitivity6.
Pancreatic Beta-Cell Dysfunction and Loss
T2DM is preceded by IR and prolonged high blood glucose levels. Pancreatic beta-cells respond by increasing insulin production. However, a continual stimulation of beta-cells leads to their dysfunction and eventual failure. Elevated glucose and fatty acid metabolism in beta-cells during hyperglycemia and high triglyceride levels increase electron transport chain activity, resulting in higher production of ROS. This oxidative stress damages mitochondria and promotes higher mitochondrial division. This accelerated division further impairs OXPHOS and increases ROS production. Progressive mitochondrial damage initiates the apoptosis pathway, resulting in beta-cell death. In genetically susceptible individuals, T2DM develops once beta-cell loss reaches a critical point16,18-21.
Mitochondria and Cell Death
Mitochondria play a crucial role in cell death processes, which can be categorized into programmed (apoptosis) or unprogrammed (necrosis). Apoptosis is initiated by activation of caspases, a group of proteases that degrade cellular substrates, triggered by intrinsic or extrinsic pathways. The intrinsic or the mitochondrial pathway can be activated by stimuli like DNA damage and ROS-mediated injury, leading to mitochondrial outer membrane permeabilization. This results in the
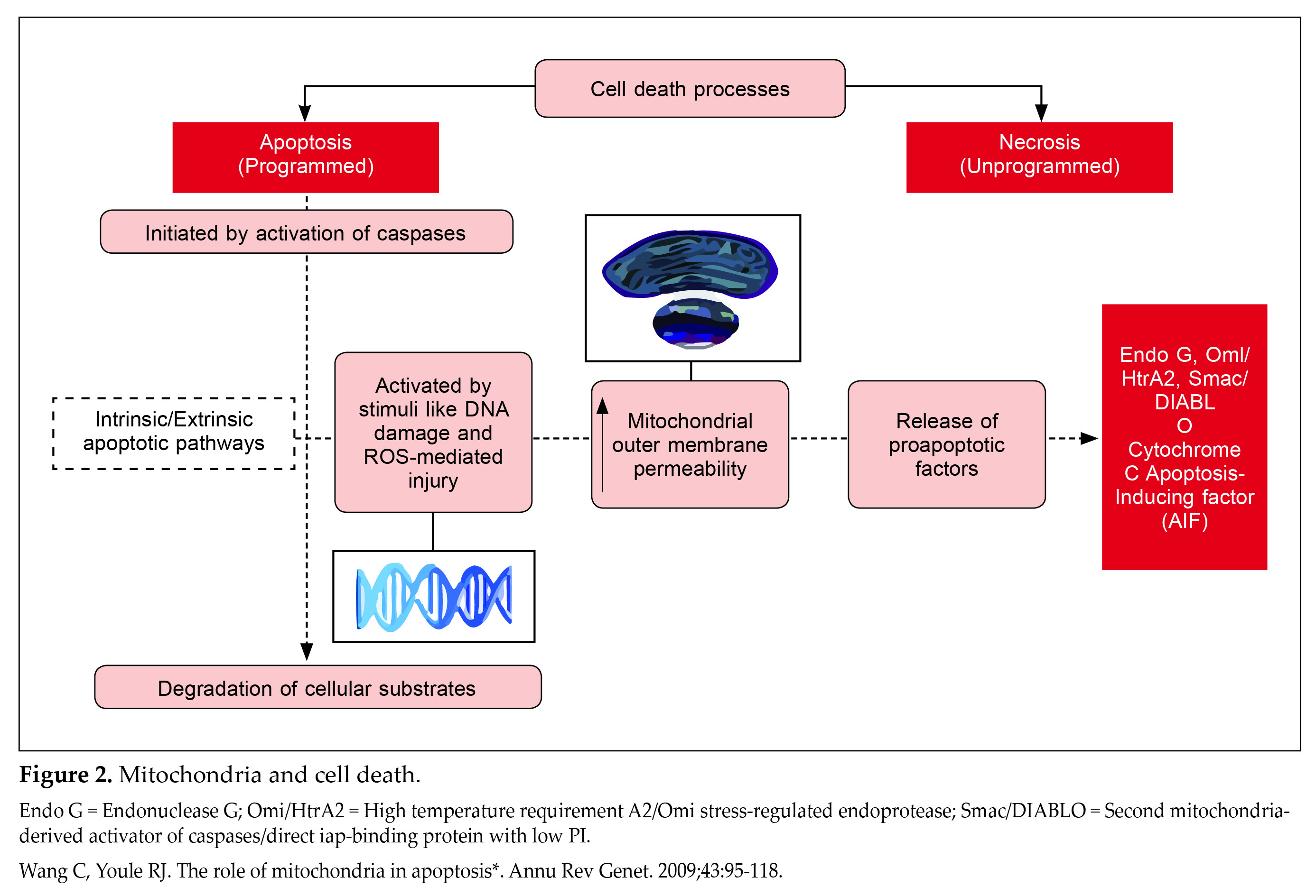
release of proapoptotic factors such as cytochrome c and apoptosis-inducing factor from the mitochondrial intermembrane space to the cytoplasm (Fig. 2)22.
Excessive ROS and intramitochondrial calcium accumulation results in the formation of mPTP in the inner mitochondrial membrane. This causes mitochondrial membrane potential collapse, ATP depletion, mitochondrial swelling, and rapid cell death (necrosis). In pancreatic beta-cells, mitochondria play a significant role in cell loss due to ROS-mediated injury23-25.
Mitophagy Inducers in the Pathogenesis and Progression of Diabetes
Mitophagy plays a critical role in maintaining mitochondrial balance and serves as an effective mechanism for clearing intracellular ROS. Recent research has shown that damaged mitochondria fuse with functional ones, leading to larger damaged mitochondria that escalate oxidative stress by releasing high levels of ROS26.
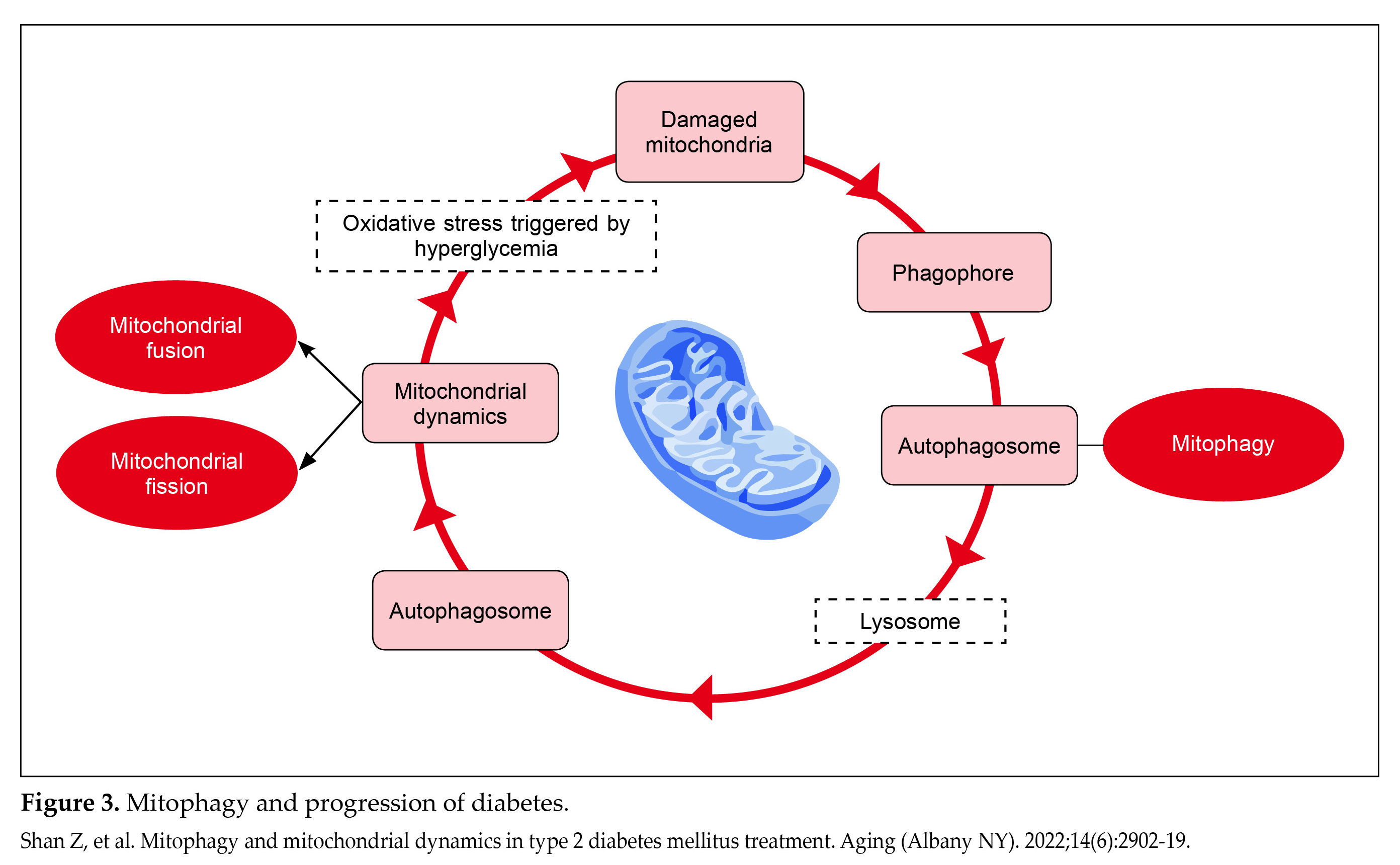
In normal mitochondrial dynamics, mitophagy is essential for maintaining a healthy mitochondrial population. It involves encapsulating damaged mitochondrial fragments through coupling with PTEN-induced putative kinase 1 (PINK1) and subsequent phosphorylation of PARKIN and ubiquitin. Phosphorylated PARKIN further facilitates outer membrane ubiquitination, promoting autophagosome formation that fuses with lysosomes for degradation28.
During hyperglycemia, oxidative stress triggers mitochondrial damage, activating mitophagy to remove damaged mitochondria through autophagosome encapsulation. Autophagosomes then fuse with lysosomes to form autolysosomes, where damaged mitochondria are degraded by acidic lysosomal hydrolases31.
However, in T2DM, pancreatic beta-cells are exposed to high glucose concentrations. This condition promotes mitochondrial fission over fusion due to increased dynamin-related protein 1 (Drp1) and degradation of protein optic atrophy 1/mitofusin (OPA1/MFN), leading to impaired mitophagy. The accumulation of dysfunctional mitochondria, along with heightened mitochondrial ROS, triggers oxidative damage to beta-cells, ultimately promoting cell death through apoptosis and contributing to IR (Fig. 3)28.
Overview and Need of Imeglimin in the Management of T2DM
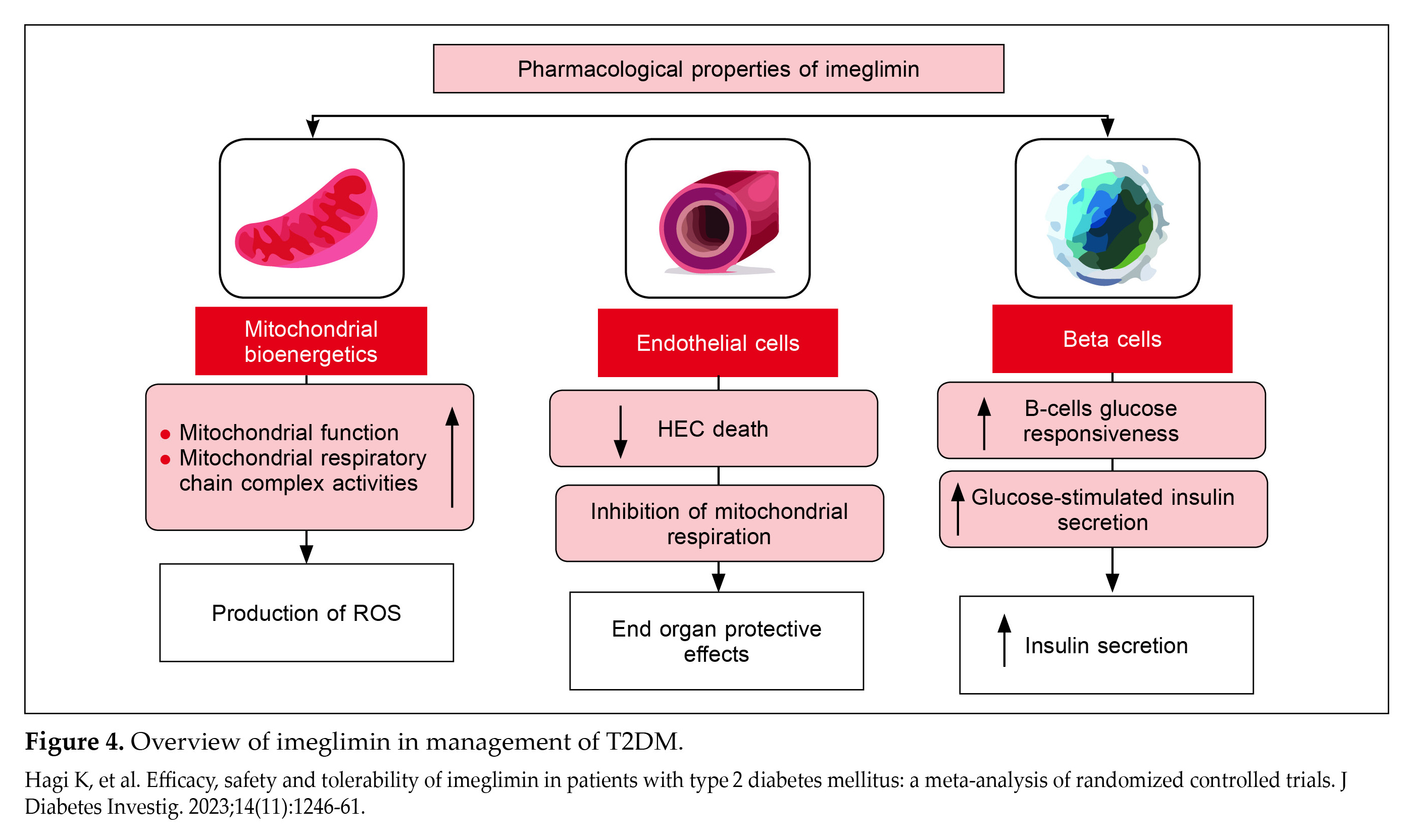
Worldwide, imeglimin is the first oral medication shown to boost insulin secretion as well as enhance insulin sensitivity to achieve optimal glucose levels. It was first approved in June 2021 in Japan for use as monotherapy and in combination therapies. Imeglimin stands out for its unique mechanism of action addressing both the physiological and molecular level of dysfunction in T2DM7.
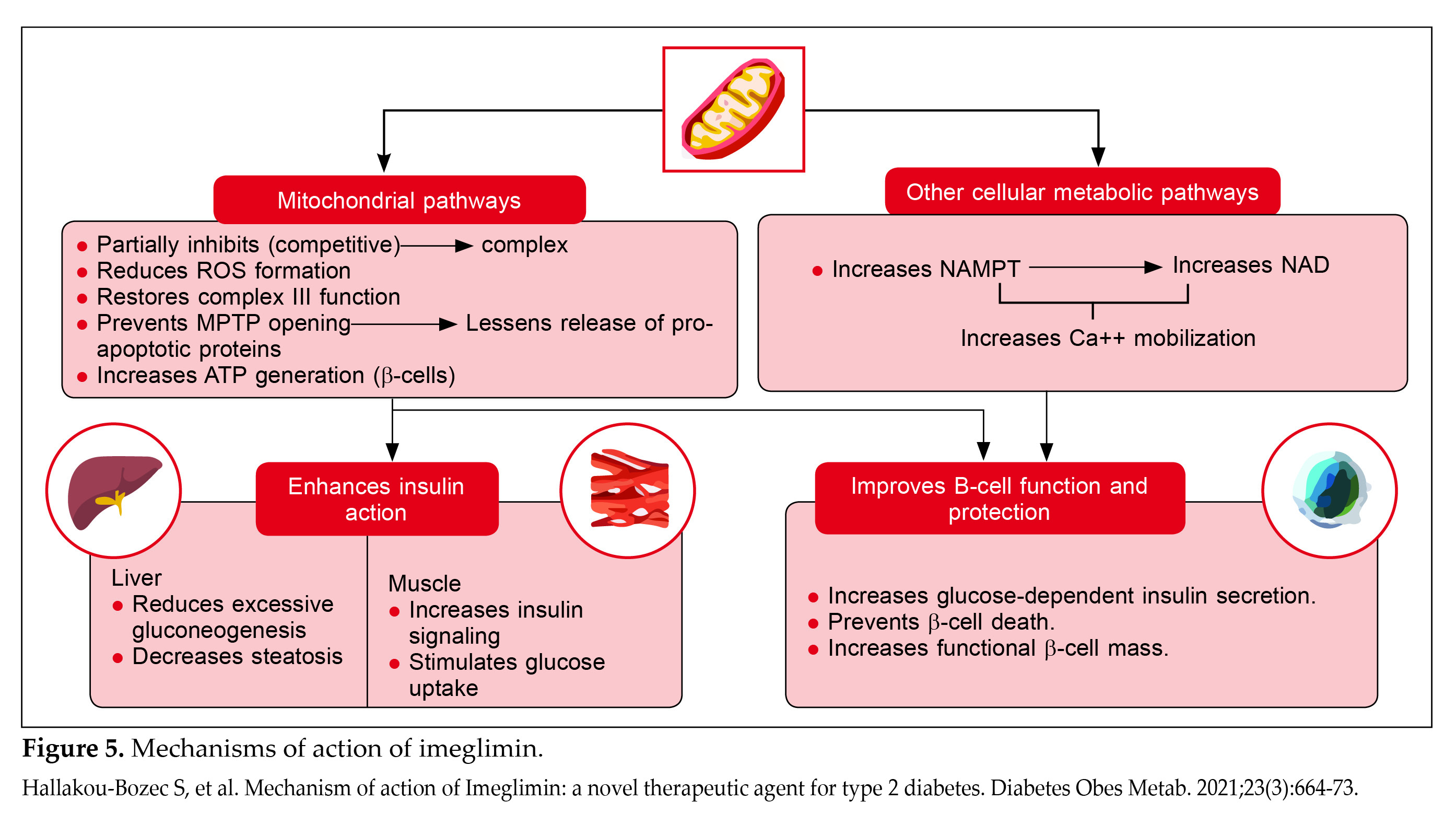
Imeglimin enhances mitochondrial bioenergetics and function, regulates respiratory chain activities, and reduces ROS production. It protects human endothelial cells without compromising mitochondrial respiration, suggesting potential end-organ protective effects. In patients with T2DM, imeglimin improves beta-cell glucose responsiveness, stimulating insulin secretion through various pathways, including the cyclic adenosine diphosphate (ADP) ribose-transient receptor potential channel (Fig. 4)6,7.
Mechanism of Action of Imeglimin
The therapeutic role of imeglimin in the management of T2DM stems from its diverse molecular mechanisms that regulate blood glucose through several pathways. These include enhancing beta-cell function and proliferation, improving insulin signaling in the liver and muscles to increase sensitivity, inhibiting gluconeogenesis, enhancing mitochondrial function, and reducing oxidative stress9,30,31.
Imeglimin enhances GSIS in islet beta-cells and also improves insulin action in the liver and skeletal muscle. These effects are driven by modulation of mitochondrial function and an increase in nicotinamide adenine dinucleotide (NAD+) generation, which supports calcium mobilization in the amplification pathway of insulin secretion in beta-cells (Fig. 5)7.
Imeglimin, compared to other antidiabetic drugs such as metformin, exhibits a unique mechanism of action. Their comparison is presented in Table 1. Sulfonylureas, including tolbutamide, act as secretagogues regardless of whether glucose levels are low or high. In contrast, the effects of imeglimin, similar to glucagon-like peptide-1 (GLP-1), are dependent on glucose levels. Additionally, while sulfonylureas lose their effect on GSIS in the presence of diazoxide, a traditional beta-cell K+-ATP channel opener, the impact of imeglimin on GSIS remains unaffected32.
Overall, the unique mechanism of imeglimin involves a wide range of actions, including direct effects on beta-cell function and mitochondrial activity, which distinguishes it from metformin.
|
Table 1. Comparison of Mechanisms of Action Between Imeglimin and Metformin7
|
|
Imeglimin
|
Metformin
|
|
In vivo (clinical)
|
|
Increased insulin secretion via glucose-stimulation
Increased insulin sensitivity
|
No effect seen on insulin secretion
No well-established elevation in insulin sensitivity
|
|
In vivo (preclinical)
|
|
Increased insulin secretion via glucose-stimulation
Increased glucose disposition leading to higher insulin sensitivity and signaling
|
No effect seen on insulin secretion
|
|
Cell and organ
|
|
Increased insulin secretion via glucose-stimulation
Protection and preservation of islet beta-cell mass
Higher glucose uptake by muscles
Decreased gluconeogenesis by hepatocytes
|
No well-established effect seen on insulin secretion via glucose stimulation
No known effect on protection and preservation of beta-cell mass
|
|
Intracellular
|
|
Competitive or partial inhibition of mitochondrial Complex I; no reduction in mitochondrial respiration; reduced formation of ROS
|
Uncompetitive inhibition of mitochondrial Complex I; reduced respiration; lower formation of ROS
|
Mitochondrial Action of Imeglimin34
Effects of Imeglimin on Mitochondrial Respiration and ROS Production
Upon assessing the effects of imeglimin on mitochondrial respiratory function using the human hepatoma cell line HepG2 and mouse primary hepatocytes, imeglimin showed concentration-dependent reductions in oxygen consumption rate under basal conditions and during ATP production. Also, imeglimin increased the mitochondrial respiration rate at lower concentrations without significant effect at higher concentrations. Furthermore, imeglimin also lowered the intracellular ROS levels, with similar effects observed at lower concentrations without further reductions at higher doses. Imeglimin therefore has been shown to exert
inhibitory effects on mitochondrial respiratory function and intracellular ROS levels33.
Effects of Imeglimin on AMPK Activity in HepG2 Cells
The effect of imeglimin on AMP-activated protein kinase (AMPK) has always been a subject of further exploration. Imeglimin has been shown to induce phosphorylation of AMPKα at threonine-172 (Thr172), indicating activation of this kinase, in a concentration-dependent manner after 3 hours of treatment, as seen in HepG2 cells and mouse primary hepatocytes. Phosphorylation of acetyl-coenzymeA carboxylase (ACC), a substrate of AMPK, was also elevated by imeglimin in HepG2 cells, confirming AMPK activation. Activation of AMPK by drugs like metformin and imeglimin improves glucose and lipid metabolism in diabetes by inhibiting lipogenesis and enhancing fatty acid oxidation, partly via liver kinase B1 (LKB1)-dependent phosphorylation34.
Overall, imeglimin has been demonstrated to activate AMPKα and stimulate ACC phosphorylation in HepG2 cells and primary hepatocytes.
Glucose-Lowering Mechanisms of Imeglimin
Improvement in Function of Endoplasmic Reticulum
The heightened demand for insulin synthesis within the endoplasmic reticulum (ER) results in ER stress. This stress leads to the accumulation of unfolded precursor proteins in the ER, activating the unfolded protein response (UPR). Initially, UPR attempts to restore proper protein folding; however, if ER stress exceeds a critical threshold, it activates the mitochondrial apoptosis pathway, ultimately leading to cell death. Moreover, oxidative stress in beta-cells exacerbates ER stress, which in turn worsens oxidative stress. ER stress induces increased intracellular calcium levels due to calcium release from the ER. Elevated intracellular calcium activates the calpain-mediated apoptosis pathway. Additionally, there is heightened calcium uptake at mitochondria-associated ER membranes (MAMs). This increased intramitochondrial calcium exacerbates oxidative stress within mitochondria and triggers apoptosis in beta-cells21,34-36.
Inhibition of hepatic glucose production
Imeglimin reduces hepatic glucose production in a dose-dependent manner by enhancing mitochondrial redox potential and lowering membrane potential in rat hepatocytes. It significantly decreases gluconeogenesis by reducing the activities of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) in rat hepatocytes37,38.
Improvement in GSIS
Imeglimin improves mitochondrial function, leading to antidiabetic effects such as enhanced GSIS and preservation of beta-cell mass39. It influences beta-cell markers like proinsulin/insulin in humans, indicating improved beta-cell function9. Nicotinamide phosphoribosyltransferase (NAMPT) regulates intracellular NAD levels crucial for cellular redox reactions40. In metabolic disorders, decreased NAD levels affect oxidative stress, apoptosis, lipid metabolism, inflammation, and IR. Imeglimin directly enhances GSIS in diabetic rodents by increasing cellular NAD via NAMPT activation, which elevates glucose-induced ATP levels9. This process involves NAD conversion to cyclic ADP ribose, essential for GSIS and calcium mobilization that triggers IR9.
Imeglimin also mitigates beta-cell apoptosis by reducing glucotoxicity through mitochondrial enhancement. It preserves beta-cell mass by inhibiting mPTP. In type 2 diabetic mice, imeglimin improves beta-cell mitochondrial integrity, enhancing ATP production crucial for insulin synthesis and secretion41,42.
Improvement in beta-cell function
Imeglimin has a favorable effect on the preservation of the number of insulin granules, the recovery of mitochondrial structure, and the reduction in apoptosis. The reduced expression of apoptosis- and inflammation-associated factors such as inflammatory cytokines may prevent cell apoptosis. A decrease in oxidative stress by imeglimin may also lead to reduced cell apoptotic-cell death and to improved cell function. The decrease in cell death by imeglimin may be closely linked with the amelioration of cell function. With the induction of apoptotic cell death, it is hard for cells to preserve the synthesis and secretion of insulin.
Although further studies should be performed, the imeglimin-mediated prevention of apoptotic-cell death may improve cell function. Briefly, the improvement in cell mitochondrial structure is likely to facilitate ATP production, enhancing-cell function. Furthermore, imeglimin-mediated improvement in structural integrity and homeostasis of ER may largely contribute to an improvement in-cell function41-43.
Enhancement of glucose uptake by the skeletal muscles
Skeletal muscle holds sway in insulin-mediated glucose disposal. IR, which diminishes glucose uptake by muscles, is a key contributor to T2DM development. Imeglimin, significantly enhances glucose uptake by muscle cells in a dose-dependent pattern. It is also important to consider that chronic administration of imeglimin over 45 days increases glucose uptake in these muscles.
The mitigation of mitochondrial dysfunction by imeglimin leads to improved insulin signaling particularly in skeletal muscle38. While imeglimin is anticipated to boost insulin resistance by enhancing the expression and function of glucose transporter 4 in muscle, this effect has yet to be demonstrated43.
Improvement in oxidative stress and IR
Oxidative stress plays an important role in the pathogenesis of diabetes and its complications. Compromised insulin signaling prompted by oxidative stress can cause IR. Imeglimin has antioxidative properties, which enables it to ameliorate free radical generation and readjust the redox state. Imeglimin reduces oxidative stress by suppressing the mitochondrial free radical generation, which improves glucose homeostasis43. Imeglimin has been shown to reduce hepatic glucose production in a dose-dependent manner by gradually increasing mitochondrial redox potential and simultaneously alleviating membrane potential in rat hepatocytes. In addition to this, imeglimin suppresses glucose production in hepatocytes43. Furthermore, imeglimin significantly inhibits gluconeogenesis by reducing levels of PEPCK and G6Pase43.
Clinical Evidence on Imeglimin Monotherapy and Combination Therapy
Clinical trials of imeglimin have consistently demonstrated significant reductions in blood glucose levels, along with a favorable safety and tolerability profile. Notably, no cases of severe hypoglycemia were reported. This clinical evidence is summarized in Tables 2.
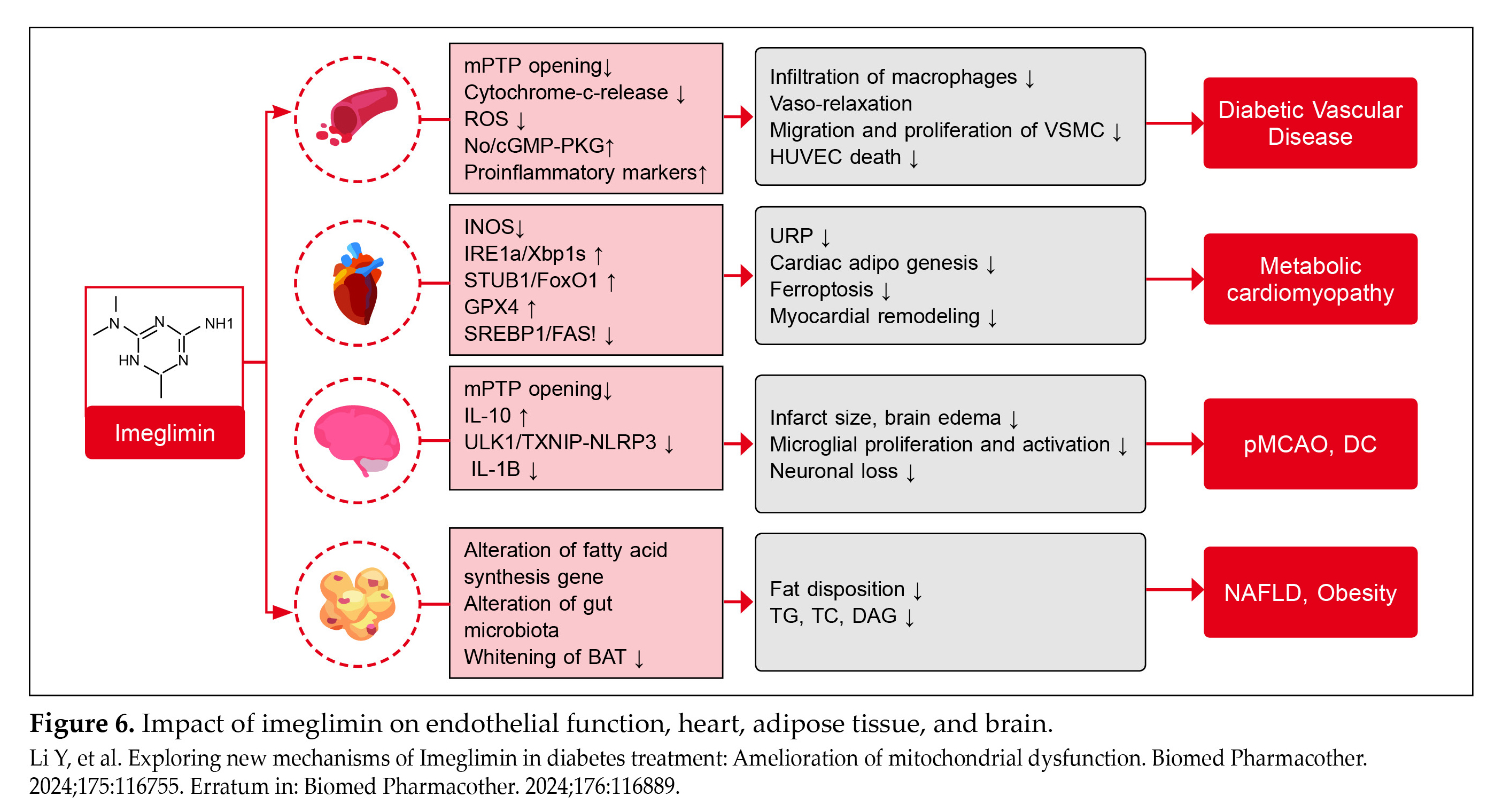
Effects of Imeglimin on Endothelial Function, Heart, Adipose Tissue, and Brain9
The multifaceted effects of imeglimin across different aspects of diabetic complications, including vascular health, cardiometabolic function, neuroprotection, and lipid metabolism are noteworthy (Fig. 6).
Diabetic Vasculopathy: Imeglimin improves diabetic vascular complications by inhibiting opening of the mPTP, reducing ROS production, enhancing nitric oxide (NO) bioavailability, and increasing production of inflammatory markers. This promotes vasodilation, reduces vascular smooth muscle cell (VSMC) migration and proliferation, and mitigates macrophage infiltration in endothelial cells.
Metabolic Cardiomyopathy: Imeglimin diminishes the cardiac metabolic stress by inhibiting inducible NO synthase (iNOS), enhancing FoxO1 degradation, and promoting GPX4 expression. This reduces cardiac lipid
|
Table 2. Clinical Trials38,43-50
|
|
Author
|
Study Design
|
Results
|
Key Insight
|
|
Li et al9
|
Treatment with imeglimin over 24 weeks
|
Significant improvement in the Quantitative Insulin Sensitivity Check Index (QUICKI) with an increase in mean QUICKI values of 0.0093 compared to those on placebo (p = 0.005) after 24 weeks.
|
Significant reductions in glucose levels and favorable results regarding safety and tolerability.
|
|
Dubourg et al50
|
Phase 2b clinical trial, 24-week study conducted in Japan, adults aged ≥20 years and older with T2DM, who were either new to treatment or had previously received one oral antidiabetic medication, were eligible to participate. This randomized, double-blind, placebo-controlled trial included parallel groups and aimed to assess the efficacy and safety of orally administered imeglimin (500 mg, 1,000 mg, or 1,500 mg, BD) compared to placebo. The primary goal was to measure the change in HbA1c levels at week 24, adjusted against the placebo. Safety assessments were conducted on all patients who received at least one dose of the study drug.
|
Imeglimin significantly decreased HbA1c (difference vs. placebo: imeglimin 500 mg −0.52% (95% CI: −0.77%, −0.27%), imeglimin 1,000 mg −0.94% (95% CI: −1.19%, −0.68%), imeglimin 1,500 mg −1.00% (95% CI: −1.26%, −0.75%); p <0.0001 for all).
A slight increase in gastrointestinal adverse effects, such as diarrhea, was observed at the 1,500 mg dose level. However, the incidence of hypoglycemia remained consistent across all treatment groups.
|
Imeglimin administered as a monotherapy was well tolerated. It effectively improved glycemic control without causing a notable increase in hypoglycemic events compared to placebo. For the subsequent phase 3 trials, imeglimin 1,000 mg BD was chosen due to its optimal efficacy and safety.
|
|
Dubourg et al44
|
Double-blind, randomized, parallel-group, placebo-controlled phase 3 trial (TIMES 1) conducted at 30 sites in Japan. Patients were randomly assigned in a 1:1 ratio to receive either oral imeglimin (1,000 mg BD) or a matched placebo for 24 weeks.
|
Compared to placebo, the adjusted mean difference in change from baseline HbA1c at week 24 was
−0.87% (95% CI −1.04 to −0.69 (9.5 mmol/mol; 95% CI −11.4 to −7.5); p < 0.0001).
In the imeglimin group, 47 patients (44.3%) reported ≥1 adverse events, compared to 48 adverse events (AEs) (44.9%) reported in the placebo group.
|
Imeglimin demonstrated significant improvement in HbA1c among Japanese patients with T2DM compared to placebo, and it exhibited a safety profile similar to that of placebo.
|
|
Dubourg et al45
|
Open-label, phase 3 trial (TIMES 2) included patients with T2DM who had inadequate control despite diet and exercise, or treatment using a single agent from various classes of antidiabetic drugs combined with diet and exercise. All patients received imeglimin orally (1,000 mg BD for 52 weeks), either as monotherapy or in combination therapy.
|
At week 52, HbA1c decreased by 0.46% with imeglimin monotherapy, by 0.56%-0.92% with imeglimin in oral combination therapy, and by 0.12% with injectable GLP-1RA combination therapy. The largest net reduction in HbA1c (0.92%) was observed in patients receiving a DPP-4
|
Imeglimin demonstrates well-tolerated long-term safety and efficacy in both monotherapy and oral combination therapy for Japanese patients with T2DM.
|
| |
|
inhibitor in combination with imeglimin.
75.5% of patients reported experiencing at least one treatment-emergent adverse event (TEAE). The majority of these events were mild or moderate in severity. Serious TEAEs, which were unrelated to the study medication, occurred in 5.6% of patients.
|
|
|
Reilhac et al46
|
Double-blind, randomized, parallel-group phase 3 trial (TIMES 3) conducted at 35 sites in Japan. Participants were randomly assigned in a 1:1 ratio to receive either imeglimin (1,000 mg BD) or a matched placebo in combination with insulin for 16 weeks. Following this, there was a subsequent 36-week open-label extension period during which all patients received imeglimin 1,000 mg BD.
|
Compared to placebo, the adjusted mean difference in change from baseline HbA1c at week 16 was −0.60% (95% CI −0.80 to −0.40); p < 0.0001). This reduction was maintained up to 52 weeks with a mean decrease of −0.64% (95% CI −0.82 to −0.46) compared to baseline.
AEs and serious AEs (SAEs) rates were similar in both treatment groups. The incidence of hypoglycemia was also comparable between the groups. In the imeglimin group, all hypoglycemic events were mild and did not require assistance.
|
Imeglimin significantly improved HbA1c in Japanese patients with insufficiently controlled T2DM treated with insulin, and it demonstrated a safety profile similar to placebo. The efficacy of imeglimin in addition to insulin was sustained over 52 weeks.
|
|
Pacini et al49
|
Double-blind, randomized, placebo-controlled study involving 33 patients with T2DM, who had a baseline HbA1c of 6.8 ± 0.1% (51 mmol/mol). These patients were either drug-naïve or had discontinued their previous metformin monotherapy for 2 weeks. They were randomly assigned to receive imeglimin 1,500 mg BD or placebo for 1 week.
|
Imeglimin treatment for 7 days significantly increased the insulin secretory response to glucose: +112% for insulin area under the curve from 0 to 45 minutes (iAUC0-45, p = 0.035), +110% for first-phase insulin secretion rate (ISR, p = 0.034), and +29% for second-phase ISR (p = 0.031). Imeglimin also improved beta-cell glucose sensitivity by +36% (p = 0.034) and showed a tendency to decrease hepatic insulin extraction by −13% (p = 0.056).
|
In patients with T2DM, imeglimin enhances beta-cell function, which likely contributes to the observed glucose-lowering effects seen in clinical trials.
|
|
Abdelhaleem et al51
|
Eight studies comprising 1,555 patients with T2DM were included in this meta-analysis.
|
Imeglimin group outperformed the control group in terms of HbA1c and FPG levels (p < 0.00001).
|
Imeglimin improved glycemic control by reducing HbA1c and FPG levels. However, no positive effects were noted in terms of IR measured by HOMA-IR or lipid parameters.
|
| |
|
Imeglimin demonstrated a favorable safety and tolerability profile, with no treatment-emergent or SAEs reported.
|
|
|
Singh et al52
|
Meta-analysis with 10 double-blind, randomized, placebo-controlled trials (RCTs) was conducted using imeglimin at a dosage of 1,000 mg BD.
|
Imeglimin 1,000 mg BD, significantly reduced HbA1c (Δ −0.9%, 95% CI −1.1 to −0.74%; p < 0.0001) compared to placebo, with no heterogeneity (I² = 0%). However, the pooled meta-analysis from all 3 RCTs (n = 574) showed a significant reduction in HbA1c with imeglimin 1,000 mg BD (Δ −0.79%, 95% CI −1.00 to −0.59%; p < 0.0001) compared to placebo, with high heterogeneity.
The tolerability profile was acceptable.
|
Imeglimin demonstrated a significant reduction in HbA1c in individuals with T2DM, accompanied by an acceptable tolerability profile.
|
|
Permana et al53
|
CENTRAL, Medline, Scopus, and ClinicalTrials.gov databases were searched using specific keywords. Continuous variables were pooled using mean difference (MD), and dichotomous variables were pooled using odds ratio (OR), both with their respective 95% CI, utilizing fixed-effect models.
|
Imeglimin at 1,000 mg BD (MD −0.90%, p < 0.00001) and 1,500 mg BD (MD −0.84%, p = 0.0003) as monotherapy demonstrated significantly greater reductions in HbA1c compared to placebo. Superiority was still maintained when given as combination therapy.
An increase in the incidence of gastrointestinal adverse events was observed with higher doses of imeglimin.
|
Imeglimin at 1,000 mg BD may provide optimal therapeutic effects for glycemic control while maintaining a favorable safety profile.
|
|
Table 3. Treatment-Emergent Adverse Effects and Other Adverse Effects with Different Dosage50
|
|
Parameters
|
Placebo
|
Imeglimin
(500 mg BD)
|
Imeglimin
(1,000 mg BD)
|
Imeglimin
(1,500 mg BD)
|
|
Any TEAEs
Mild
Moderate
Severe
|
51 (68.0)
49 (65.3)
6 (8.0)
0
|
51 (68.0)
51 (68.0)
3 (4.0)
0
|
46 (62.2)
44 (59.5)
4 (5.4)
4 (5.4)
|
55 (73.3)
52 (69.3)
9 (12.0)
1 (1.3)
|
|
Drug-related TEAEs
|
6 (8.0)
|
4 (5.3)
|
4 (5.4)
|
18 (24.0)
|
|
Serious TEAEs
Bradycardia
Clavicle fracture
Meniscus injury
|
0
0
1 (1.3)
|
0
0
0
|
1 (1.4)
1 (1.4)
0
|
0
0
0
|
|
TEAE leading to discontinuation
|
10 (13.3)
|
2 (2.7)
|
5 (6.8)
|
5 (6.7)
|
accumulation, improves myocardial remodeling, and supports metabolic health in cardiomyopathy.
Diabetic Neuroinflammation: Imeglimin reduces brain injury and neurological deficits by inhibiting mPTP in neurons and astrocytes. It increases production of IL-10 (anti-inflammatory cytokine), reduces infarct size and brain edema, and suppresses inflammatory pathways like TXNIP–NLRP3, thereby protecting neurons and promoting neuroprotection.
Diabetic Lipid Metabolism Disorder: Imeglimin alters fatty acid synthesis genes and enhances the Akkermansia genus in the gut microbiome. This reduces lipid deposition in brown adipose tissue, lowers liver triglycerides, cholesterol, and diacylglycerol levels, alleviating hepatic steatosis, and improving onset of conditions like nonalcoholic fatty liver disease (NAFLD) and obesity.
Indications, Contraindications and Dosage
Indications56
- T2DM inadequately controlled with diet and exercise alone.
Contraindications55
- Patients with a history of hypersensitivity reaction to the active substance or to any of the excipients.
- Patients with renal dysfunction with estimated glomerular filtration rate (eGFR) <45 mL/min/1.73 m2 (including dialysis patients).
- Pregnant and lactating women.
- Patients with hepatic dysfunction Child-Pugh Class C.
Posology of Imeglimin56
- Available as 500 and 1,000 mg tablets.
- Dose 1,000 mg twice a day post-meal.
Safety Profile of Imeglimin
The safety profile appears favorable, as evidenced by the lack of significant AEs, cardiovascular issues, or increased incidences of hypoglycemia among patients treated with imeglimin49,53. A comprehensive meta-analysis of safety and tolerability profile of imeglimin observed that when compared to placebo, there was no significant difference observed in discontinuation of imeglimin treatment, treatment discontinuation due to adverse events, treatment withdrawal due to occurrence of at least one AE or serious AE (SAE). Imeglimin, whether used alone or in combination with other treatments, was not associated with an increased risk of AEs or hypoglycemia7. A 52-week phase 3 trial by Dubourg et al observed that among patients administered with imeglimin monotherapy, nausea was observed in 6.7% of patients, followed by constipation (3.7%), gastroesophageal reflux disease (2.2%), vomiting (0.7%), and diarrhea (0.3%)45.
Imeglimin was better tolerated at the dose of 1,000 mg BD59. A randomized trial by Dubourg et al evaluated imeglimin 2,250 mg, imeglimin 6,000 mg, moxifloxacin 400 mg, and placebo. It was observed that throughout the course of the study, there were no QT/QTc prolongation for both therapeutic and supratherapeutic doses of imeglimin when compared to placebo. In addition to this, imeglimin did not exert any relevant effect on heart rate or PR or QRS intervals57.
Progress in Clinical Research on Imeglimin
Constant exploration of new molecules in medicine is crucial for addressing unmet needs, enhancing efficacy, minimizing side effects, and managing resistance, ultimately improving patient care. Understanding how different tissues and cell types respond to imeglimin is vital, given its complexities across various biological contexts.
A notable aspect of imeglimin is that it does not affect mitochondrial bioenergetics in endothelial cells, which starkly contrasts with its effects in liver tissue. In the liver, imeglimin significantly alters the activity of various electron transport chain complexes, along with changes in gene expression and carnitine levels that indicate enhanced fatty acid oxidation. This suggests that the influence of imeglimin on respiratory chain and fatty acid metabolism may be more significant in dysfunctional mitochondria compared to healthy ones60.
Additionally, mitochondrial respiration and oxygen consumption assessments in liver samples from high fat-high sucrose diet (HFHSD) mice, conducted in the presence of insulin, help explain the observed mitochondrial effects, contrasting with endothelial cell experiments lacking insulin. Notably, imeglimin treatment reduced ROS production generated by reverse electron transport in complex I of endothelial cells and in succinate-energized mitochondria from HFHSD mice. This indicates that imeglimin may correct mitochondrial defects, particularly in functionally impaired scenarios, rather than affecting energetics under normal conditions. These findings suggest the need for further investigations into the effects of imeglimin across different tissues, taking into account variations in cell types and experimental models that may influence mitochondrial responses59.
In India, there are currently 16 registered clinical trials evaluating the safety and efficacy of imeglimin (at doses of 500 and 1,000 mg BD), including in combination with metformin and sitagliptin; however, some of these are listed as ‘not yet recruiting’ and may not be actively ongoing58.
A global clinical trial, the DIGNITY trial (ClinicalTrials.gov identifier: NCT05366868), currently in the recruitment phase, is evaluating the long-term durability of glycemic control over a 3-year period in patients with type 2 diabetes managed with diet, exercise, and oral hypoglycemic monotherapy59.
Conclusion
Imeglimin is a novel oral antidiabetic agent that targets mitochondrial bioenergetics to improve both insulin secretion and insulin sensitivity. Approved in Japan and China in 2021 and introduced in India in 2022, it enhances glucose-stimulated insulin secretion, preserves beta-cell mass, reduces hepatic glucose output, and improves insulin signaling in the liver and muscles. At the molecular level, it corrects mitochondrial dysfunction by modulating respiratory chain activity and reducing oxidative stress. Unlike other drug classes, imeglimin addresses both insulin resistance and secretion. Clinical trials have shown it to be effective in lowering A1c by 0.5%-0.65% as monotherapy or with agents like sitagliptin or metformin, with a favorable safety profile and mainly mild gastrointestinal side effects.
References
- World Health Organization. Diabetes. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed June 28, 2024.
- International Diabetes Federation. Facts & figures. Available from: https://idf.org/about-diabetes/diabetes-facts-figures/. Accessed August 9, 2024.
- Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK,et al; ICMR–INDIAB Collaborative Study Group. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5(8):585-96. Erratum in: Lancet Diabetes Endocrinol. 2017;5(8):e5.
- Dludla PV, Mabhida SE, Ziqubu K, Nkambule BB, Mazibuko-Mbeje SE, Hanser S, et al. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J Diabetes. 2023;14(3):130-46.
- Prasun P. Role of mitochondria in pathogenesis of type 2 diabetes mellitus. J Diabetes Metab Disord. 2020;19(2):2017-22.
- Hagi K, Nitta M, Watada H, Kaku K, Ueki K. Efficacy, safety and tolerability of imeglimin in?patients with type 2 diabetes mellitus: a?meta-analysis of randomized controlled trials. J Diabetes Investig. 2023;14(11):1246-61.
- Hallakou-Bozec S, Vial G, Kergoat M, Fouqueray P, Bolze S, Borel AL, et al. Mechanism of action of Imeglimin: a novel therapeutic agent for type 2 diabetes. Diabetes Obes Metab. 2021;23(3):664-73.
- Lamb YN. Imeglimin hydrochloride: first approval. Drugs. 2021;81(14):1683-90.
- Li Y, Lou N, Liu X, Zhuang X, Chen S. Exploring new mechanisms of Imeglimin in diabetes treatment: Amelioration of mitochondrial dysfunction. Biomed Pharmacother. 2024;175:116755. Erratum in: Biomed Pharmacother. 2024;176:116889.
- Islam MS. Stimulus-secretion coupling in beta-cells: from basic to bedside. Adv Exp Med Biol. 2020;1131:943-63.
- MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab. 2005;288:E1-15.
- Schuit F, De Vos A, Farfari S, Moens K, Pipeleers D, Brun T, et al. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem. 1997;272(30):18572-9.
- Olsson AH, Yang BT, Hall E, Taneera J, Salehi A, Dekker Nitert M, et al. Decreased expression of genes involved in oxidative phosphorylation in human pancreatic islets from patients with type 2 diabetes. Eur J Endocrinol. 2011;165(4):589-95.
- Sergi D, Naumovski N, Heilbronn LK, Abeywardena M, O’Callaghan N, Lionetti L, et al. Mitochondrial (Dys)function and insulin resistance: from pathophysiological molecular mechanisms to the impact of diet. Front Physiol. 2019;10:532.
- Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med. 2010;16(4):400-2. 22. Petersen MC, Shulman GI. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol Sci. 2017;38(7):649-65.
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1and NRF1. Proc Natl Acad Sci U S A. 2003;100(14):
8466-71.
- Burkart AM, Tan K, Warren L, Iovino S, Hughes KJ, Kahn CR, et al. Insulin resistance in human iPS cells reduces mitochondrial size and function. Sci Rep. 2016;6:22788.
- Hurrle S, Hsu WH. The etiology of oxidative stress in insulin resistance. Biomed J. 2017;40(5):257-62.
- Galloway CA, Lee H, Nejjar S, Jhun BS, Yu T, Hsu W, et al. Transgenic control of mitochondrial fission induces mitochondrial uncoupling and relieves diabetic oxidative stress. Diabetes. 2012;61(8):2093-104.
- Zhang P, Li T, Wu X, Nice EC, Huang C, Zhang Y. Oxidative stress and diabetes: antioxidative strategies. Front Med. 2020;14(5):583-600. Archer SL. Mitochondrial dynamics–mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369(23):2236-51.
- Hasnain SZ, Prins JB, McGuckin MA. Oxidative and endoplasmic reticulum stress in β-cell dysfunction in diabetes. J Mol Endocrinol. 2016;56(2):R33-54.
- Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95-118.
- McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5(4):a008656.
- Parsons MJ, Green DR. Mitochondria in cell death. Essays Biochem. 2010;47:99-114.
- Armstrong JA, Cash NJ, Ouyang Y, Morton JC, Chvanov M, Latawiec D, et al. Oxidative stress alters mitochondrial bioenergetics and modifies pancreatic cell death independently of cyclophilin D, resulting in an apoptosis-to-necrosis shift. J Biol Chem. 2018;293(21):8032-47.
- Dorn GW 2nd, Kitsis RN. The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble. Circ Res. 2015;116(1):167-82.
- Sidarala V, Pearson GL, Parekh VS, Thompson B, Christen L, Gingerich MA, et al. Mitophagy protects β cells from inflammatory damage in diabetes. JCI Insight. 2020; 5(24):e141138.
- Shan Z, Fa WH, Tian CR, Yuan CS, Jie N. Mitophagy and mitochondrial dynamics in type 2 diabetes mellitus treatment. Aging (Albany NY). 2022;14(6):2902-19.
- Bhansali S, Bhansali A, Dutta P, Walia R, Dhawan V. Metformin upregulates mitophagy in patients with T2DM: a randomized placebo-controlled study. J Cell Mol Med. 2020; 24(5):2832-46.
- Yaribeygi H, Maleki M, Sathyapalan T, Jamialahmadi T, Sahebkar A. Molecular mechanisms by which imeglimin improves glucose homeostasis. J Diabetes Res. 2020;2020:8768954.
- Pirags V, Lebovitz H, Fouqueray P. Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes Metab. 2012;14(9):
852-8.
- Proks P, Reimann F, Green N, Gribble F, Ashcroft F.?Sulfonylurea stimulation of insulin secretion. Diabetes.?2002;51 Suppl 3:
368-76.
- Hozumi K, Sugawara K, Ishihara T, Ishihara N, Ogawa W. Effects of imeglimin on mitochondrial function, AMPK activity, and gene expression in hepatocytes. Sci Rep. 2023;13(1):746.
- Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem. 2012;81:767-93. Fujii J, Homma T, Kobayashi S, Seo HG. Mutual interaction between oxidative stress and endoplasmic reticulum stress in the pathogenesis of diseases specifically focusing on non-alcoholic fatty liver disease. World J Biol Chem. 2018;9(1):1-15.
- Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21(3):396-413.
- Ly LD, Xu S, Choi SK, Ha CM, Thoudam T, Cha SK, et al. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp Mol Med. 2017;49(2):e291. 52. Momeni HR. Role of calpain in apoptosis. Cell J. 2011;13(2):65-72
- Vial G, Lamarche F, Cottet-Rousselle C, Hallakou-Bozec S, Borel AL, Fontaine E. The mechanism by which imeglimin inhibits gluconeogenesis in rat liver cells. Endocrinol Diabetes Metab. 2021;4(2):e00211.
- Fouqueray P, Leverve X, Fontaine E, Baquié M, Wollheim C. Imeglimin—A new oral anti-diabetic that targets the three key defects of type 2 diabetes.?J Diabetes Metab.?2011;2:126.
- Li J, Inoue R, Togashi Y, Okuyama T, Satoh A, Kyohara M, et al. Imeglimin ameliorates β -cell apoptosis by modulating the endoplasmic reticulum homeostasis pathway.?Diabetes.?2022;71(3):424-39.
- Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism.?Nat Rev Endocrinol.?2015;11(9):535-46.
- Lablanche S, Tubbs E, Cottet-Rousselle C, Lamarche F, Moisan A, Persoons V, et al. Imeglimin protects INS-1 cells and human islets against high glucose– and high fructose–induced cell death by inhibiting the mitochondrial PTP opening.?Diabetes.?2018;67(Suppl 1):81-OR.
- Sanada J, Obata A, Fushimi Y, Kimura T, Shimoda M, Ikeda T, et al. Imeglimin exerts favorable effects on pancreatic β-cells by improving morphology in mitochondria and increasing the number of insulin granules.?Sci Rep.?2022;12:13220.
- Yanai H, Adachi H, Hakoshima M, Katsuyama H. Glucose-lowering effects of imeglimin and its possible beneficial effects on diabetic complications. Biology (Basel). 2023;12(5):726.
- Dubourg J, Fouqueray P, Thang C, Grouin JM, Ueki K. Efficacy and safety of imeglimin monotherapy versus placebo in Japanese patients with type 2 diabetes (TIMES 1): a double-blind, randomized, placebo-controlled, parallel-group, multicenter phase 3 trial. Diabetes Care. 2021;44(4):952-9.
- Dubourg J, Fouqueray P, Quinslot D, Grouin JM, Kaku K. Long-term safety and efficacy of imeglimin as monotherapy or in combination with existing antidiabetic agents in Japanese patients with type 2 diabetes (TIMES 2): a 52-week, open-label, multicentre phase 3 trial. Diabetes Obes Metab. 2022;24(4):
609-19.
- Reilhac C, Dubourg J, Thang C, Grouin JM, Fouqueray P, Watada H. Efficacy and safety of imeglimin add-on to insulin monotherapy in Japanese patients with type 2 diabetes (TIMES 3): a randomized, double-blind, placebo-controlled phase 3 trial with a 36-week open-label extension period. Diabetes Obes Metab. 2022;24(5):838-48.
- Fouqueray P, Pirags V, Inzucchi SE, Bailey CJ, Schernthaner G, Diamant M, et al.?The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy.?Diabetes Care. 2013;36(3):565-8.
- Fouqueray P, Pirags V, Diamant M, Schernthaner G, Lebovitz HE, Inzucchi SE, et al. The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with sitagliptin monotherapy. Diabetes Care. 2014;37(7):1924-30.
- Pacini G, Mari A, Fouqueray P, Bolze S, Roden M. Imeglimin increases glucose-dependent insulin secretion and improves β-cell function in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(6):541-5.
- Dubourg J, Ueki K, Grouin JM, Fouqueray P. Efficacy and safety of imeglimin in Japanese patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled, dose-ranging phase 2b trial. Diabetes Obes Metab. 2021;23(3):800-10.
- Abdelhaleem IA, Salamah HM, Alsabbagh FA, Eid AM, Hussien HM, Mohamed NI, et al. Efficacy and safety of imeglimin in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials. Diabetes Metab Syndr. 2021;15(6):102323.
- Singh AK, Singh A, Singh R, Misra A. Efficacy and safety of imeglimin in type 2 diabetes: a systematic review and meta-analysis of randomized placebo-controlled trials. Diabetes Metab Syndr. 2023;17(2):102710.
- Permana H, Soetedjo NNM, Yanto TA, Tendean M, Hariyanto TI, Suastika K. Different doses of imeglimin for management of type 2 diabetes mellitus: a systematic review, meta-analysis, and meta-regression of randomized clinical trials. Expert Rev Endocrinol Metab. 2024;19(1):89-98.
- A clinical study to assess the efficacy and safety of Imeglimin Tablets 1000 mg in patients with diabetes. Available from: https://ctri.nic.in/Clinicaltrials/pmaindet2. hp?EncHid=ODEwMzM=&Enc=&userName=imeglimin. Last accessed May 2, 2025.
- Kalra S, Bhattacharya S, Shaikh S. Imeglimin: Finding a place in modern diabetes pharmacotherapeutics. Ind J Clin Practice. 2022;33(5):8-10.
- Dubourg J, Perrimond-Dauchy S, Felices M, Bolze S, Voiriot P, Fouqueray P. Absence of QTc prolongation in a thorough QT study with imeglimin, a first in class oral agent for type 2 diabetes mellitus. Eur J Clin Pharmacol. 2020;76(10):1393-400.
- A clinical study to assess the efficacy and safety of Imeglimin Tablets 1000 mg in patients with diabetes. Available from: https://ctri.nic.in/Clinicaltrials/pmaindet2.hp?EncHid=ODEwMzM=&Enc=&userName=imeglimin. Last accessed on: 2nd May 2025.
- Durable effect of?imeglimin?on the glycemic control in patients with type 2 diabetes mellitus. Available from: ClinicalTrials.gov. Accessed July 7, 2024.
- Konkwo C, Perry RJ. Imeglimin: current development and future potential in type 2 diabetes. Drugs. 2021;81(2):
185-190.
|