Abstract
Background: Diabetes is associated with almost twofold increased risk of cardiovascular diseases (CVD). The present case-based questionnaire survey evaluated the treatment pattern and clinical experience of healthcare professionals in prescribing glimepiride/metformin fixed-dose combination (FDC) to type 2 diabetes mellitus (T2DM) patients with CVD or those patients who are at risk of CVD in the Indian settings. Material and methods: A retrospective, multicenter, observational, case-based questionnaire survey was conducted in Indian healthcare centers using medical records of patients having T2DM, with CVD or are at risk of CVD, who were prescribed any strength of glimepiride/metformin FDC. Data was collected from the patients’ medical records and was analyzed using statistical tests. Results: A total of 680 patients with T2DM with CVD or at risk of CVD were included in this study. Mean duration of diabetes in the patients was 5.7 ± 4.8 years. About 68.5% patients had hypertension, 47.9% had dyslipidemia, 25.4% had coronary artery disease (CAD), 3.6% had transient ischemic attack (TIA), 4.8% had peripheral arterial disease (PAD) and 2.9% had heart failure. Around 18.1% patients had CVD after diabetes was diagnosed, while 81.9% presented with cardiovascular (CV) issues at the time of diabetes diagnosis. All patients received glimepiride/metformin FDC as first-line therapy. About 68.2% patients on glimepiride/metformin FDC had blood pressure within optimal limits. A large proportion of patients had improvement in glycemic parameters. Weight change was noted in 18.4% of the patients overall. Of these, 59.2% had reduction in weight. There were no major adverse events and treatment efficacy and tolerability were reported as good to excellent for 94.6% and 92.9% patients, respectively. Conclusion: This case-based questionnaire survey demonstrates the usage pattern of various strengths of glimepiride/metformin FDC and the clinicians’ practice approach regarding early initiation of this combination in Indian patients with diabetes who have or are at risk of CVD.
Keywords: Type 2 diabetes, CVD, glimepiride/metformin combination, combination therapy
Diabetes and raised levels of blood glucose, even below the threshold for diabetes diagnosis, are linked with almost twofold increased risk of cardiovascular diseases (CVD). It has been reported that the prevalence of CVD is around 32% and that of coronary artery disease (CAD) is about 21% among adults living with diabetes in high- and middle-income countries.1
The most common forms of CVD tied to diabetes include coronary heart disease, cerebrovascular disease, peripheral artery disease (PAD) and congestive heart failure.1 Diabetes, and even lesser degree of dysglycemia, are associated with adverse cardiovascular (CV) outcomes.2
Across the spectrum of fasting plasma glucose (FPG), glycated hemoglobin (HbA1c) or 2-hour glucose test results, each standard deviation (SD) is tied to a
6% to 20% increased risk of CV events.1 Diabetes tends to increase the risk of CVD by several mechanisms, such as insulin resistance, inflammation, endothelial dysfunction and the adverse effects of glucose on microvasculature. Raised blood glucose levels are also linked with hypertension, dyslipidemia and obesity. Smoking and low levels of physical activity also contribute to increased CVD risk.1
Metformin is a well-established first-line treatment of type 2 diabetes mellitus (T2DM) and is effective both as monotherapy and in combination with other hypoglycemic agents. Recent data from CV and renal outcomes trials have shown additional protection from complications for some high-risk patients with other hypoglycemic medications. So, use of newer antihyperglycemic drugs with CV benefits can be considered in high-risk patients.3 The 10-year follow-up of the United Kingdom Prospective Diabetes Study (UKPDS) noted persistent benefit after metformin therapy in overweight T2DM patients, with significant risk reductions persisting for any diabetes-related end point, myocardial infarction (MI) and death from any cause over the long-term.4
Modern sulfonylureas (SUs), like glimepiride, are CV-neutral. They can maintain myocardial ischemic preconditioning with lesser CV side effects in comparison with conventional SUs. Additionally, these SUs do not seem to be associated with all-cause or CV mortality, or with an increased risk of MI or stroke. Thus, they are cardiac-friendly and can be safely used in diabetes patients with CV risk, MI or stroke.5 An International expert group advocates that on account of their safety, efficacy as well as low-cost, modern SUs could be the drugs of choice for the treatment of diabetes. The group endorses the use of newer SUs like glimepiride on account of their CV safety. The International Diabetes Federation also says that SUs have neutral effects on major CV events. Glimepiride, in particular, has been found to be associated with reduced mortality in diabetes patients with CAD, compared with other SUs.5
Experts are also of the opinion that because modern SUs have been used as comparators in other cardiovascular outcome trials (CVOTs), CVOTs with SUs are not needed.6 For instance, the CAROLINA trial compared linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, with glimepiride in terms of major adverse CV outcomes in diabetes patients and noted the drugs to exert a similar effect with regard to a risk of a composite CV outcome.7
The CV-neutral profile of metformin and modern SUs in diabetes patients has opened new avenues for the management of patients with diabetes and CVD. There is a need for physician opinion on the use of glimepiride/metformin FDC in diabetes patients with CVD. A case-based questionnaire survey was thus designed to evaluate the demography, treatment pattern including duration and various dosages of glimepiride/metformin FDC in T2DM patients with CVD or at risk of CVD.
MATERIAL AND METHODS
Study Design
This was a retrospective, multicenter, observational, case-based questionnaire survey. It was conducted with 86 healthcare professionals (HCPs) across different centers in India between July 2020 and May 2021. The study protocol was designed in accordance with the principles of the Declaration of Helsinki.
Study Population
Patients of both sexes, aged above 18 years, who received a glimepiride/metformin FDC in any strength, for the treatment of T2DM were recruited in the study. Patients with existing CVD comorbidities or at risk of CVD were included in the study.
Data Collection
A case report format was developed to evaluate the clinical utilization pattern of different strengths of glimepiride/metformin FDC in addition to other oral hypoglycemic agents (OHAs) in diabetes patients. The questionnaire was sent to 86 HCPs across India via an online portal. Link to the portal was shared through e-mail. Questions regarding demographic characteristics, such as age, sex, body mass index (BMI) and medical history; presence of CVD; duration of diabetes; biochemical measures, including FPG, postprandial plasma glucose (PPG) and HbA1c levels; comorbidities; antidiabetic drugs taken; antidiabetic drug up-titrations and down-titrations; weight changes; hypoglycemic episodes and other adverse events during treatment, were included in the questionnaire. An online portal was developed where the HCPs were required to fill in the information. A descriptive analysis was performed with the data provided on the portal.
Statistical Analysis
All continuous variables are expressed as mean ± SD or median with interquartile range as per the distribution of data. Categorical variables are expressed as number and their respective percentage. Differences in binary and
ordinal variables between two independent groups were analyzed by the exact Chi-square test. All the reported p-values are two-sided and p-values <0.05 are considered to indicate statistical significance. All data entries and statistical analyses were performed by using SPSS@ Version 23.0 software.
RESULTS
A total of 680 patients with T2DM with CVD or at risk of CVD were included in this retrospective observational questionnaire-based analysis. The mean (±SD) age of patients was 49.2 (±12.9) years (range 19-84 years). Patient demographics are summarized in Table 1.
Mean duration of diabetes in the patients was 5.7 ± 4.8 years (range 0.0-25.5 years). A vast majority of patients had diabetes duration of 1 to 5 years (n = 407), followed by 6 to 10 years (n = 164), 11 to 15 years (n = 46), <1 year (n = 29), 16 to 20 years (n = 22) and >20 years (n = 12).
A total of 466 (68.5%) patients had hypertension, 326 (47.9%) had dyslipidemia, 173 (25.4%) had CAD, 25 (3.6%)
had transient ischemic attack (TIA), 33 (4.8%) had PAD and 20 (2.9%) had heart failure (Table 2).
A total of 123 (18.1%) patients had CVD after diabetes was diagnosed, while 557 (81.9%) presented with CV issues at the time of diabetes diagnosis.
All patients included in the study received glimepiride/metformin FDC as first-line therapy. The most commonly prescribed regimen was glimepiride 0.5 mg/metformin 500 mg (31.8%) (Table 3). A majority of the patients (n = 407) received glimepiride/metformin FDC therapy early during the course of the disease, i.e., a total of 407 patients with diabetes duration of 1 to 5 years were prescribed combination therapy.
Dose titration was done in 277 patients. Up-titration was done in 239 patients (35.1%) while down-titration was done in 38 patients (5.6%). In all, 392 (57.6%) patients received other OHAs along with glimepiride/metformin FDC. These included sodium-glucose cotransporter-2 (SGLT2) inhibitors (n = 123 [18.1%]), DPP-4 inhibitors (n = 241 [35.4%]), alpha-glucosidase inhibitors (AGIs) (n = 71 [10.4%]), thiazolidinediones (n = 18 [2.6%]) and glucagon-like peptide-1 (GLP-1) agonists (n = 3 [0.4%]). Around 7.9% patients also received insulin therapy. Very few patients experienced hypoglycemia (n = 36 [5.3%]).
Majority of the patients on glimepiride/metformin FDC therapy had blood pressure (BP) within optimal limits (n = 464 [68.2%]). Weight change was evident in 125 patients (18.4%) overall. Majority of these patients
(n = 74 [59.2%]) had reduction in weight. Mean HbA1c at study initiation was 8.3% ± 1.3% and decreased to 7.2% ± 3.1% after treatment with glimepiride/metformin FDC therapy. Mean FPG prior to treatment was 174.1 ±
46.4 mg/dL and declined to 124.9 ± 28.9 mg/dL after treatment. Likewise, mean PPG before and after
|
Table 1. Patient Demographics
|
|
Variable
|
Mean ± SD/n (%)
|
|
Age (years)
|
49.2 ± 12.9
|
|
Weight (kg)
|
73.2 ± 10.4
|
|
BMI (kg/m2)
|
27.7 ± 3.9
|
|
Gender
|
Male - 446 (65.6)
Female - 231 (34)
Other - 3 (0.4)
|
SD = Standard deviation; BMI = Body mass index.
|
Table 2. Comorbidities with T2DM
|
|
Comorbidities
|
Patients N (%)
|
|
Hypertension
|
466 (68.5)
|
|
Dyslipidemia
|
326 (47.9)
|
|
CAD
|
173 (25.4)
|
|
TIA
|
25 (3.6)
|
|
PAD
|
33 (4.8)
|
|
Heart failure
|
20 (2.9)
|
T2DM = Type 2 diabetes mellitus; CAD = Coronary artery disease; TIA = Transient ischemic attack; PAD = Peripheral artery disease.
|
Table 3. Different Strengths of Glimepiride/Metformin FDC Prescribed to Study Participants
|
|
Glimepiride/Metformin FDC regimen
|
Patients N (%)
|
|
Glimepiride 0.5 mg/Metformin 1000 mg
|
14 (2.1)
|
|
Glimepiride 1 mg/Metformin 1000 mg
|
44 (6.5)
|
|
Glimepiride 2 mg/Metformin 1000 mg
|
53 (7.8)
|
|
Glimepiride 3 mg/Metformin 1000 mg
|
10 (1.5)
|
|
Glimepiride 4 mg/Metformin 1000 mg
|
7 (1)
|
|
Glimepiride 0.5 mg/Metformin 500 mg
|
216 (31.8)
|
|
Glimepiride 1 mg/Metformin 500 mg
|
175 (25.7)
|
|
Glimepiride 2 mg/Metformin 500 mg
|
119 (17.5)
|
|
Glimepiride 1 mg/Metformin 850 mg
|
4 (0.6)
|
|
Glimepiride 2 mg/Metformin 850 mg
|
15 (2.2)
|
|
Glimepiride 3 mg/Metformin 850 mg
|
23 (3.4)
|
|
Total
|
680 (100)
|
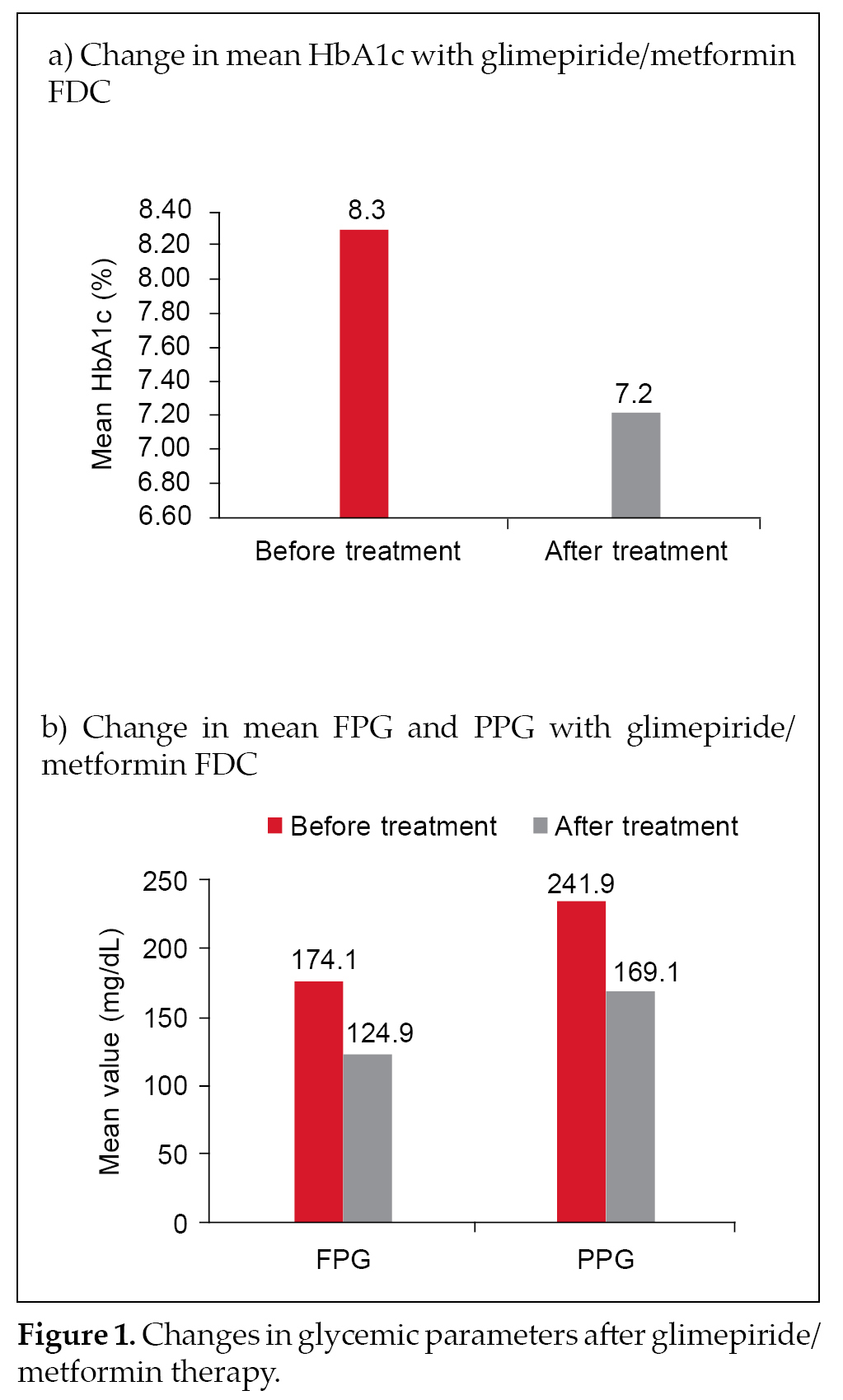
treatment was 241.9 ± 56.7 mg/dL and 169.1 ± 34.7 mg/dL,respectively. Changes in the three glycemic parameters are depicted in Figure 1 a and b.
No major adverse events were noted during the study duration. Minor adverse events included flatulence, heartburn, nausea, occasional dyspepsia, reduced appetite and occasional diarrhea.
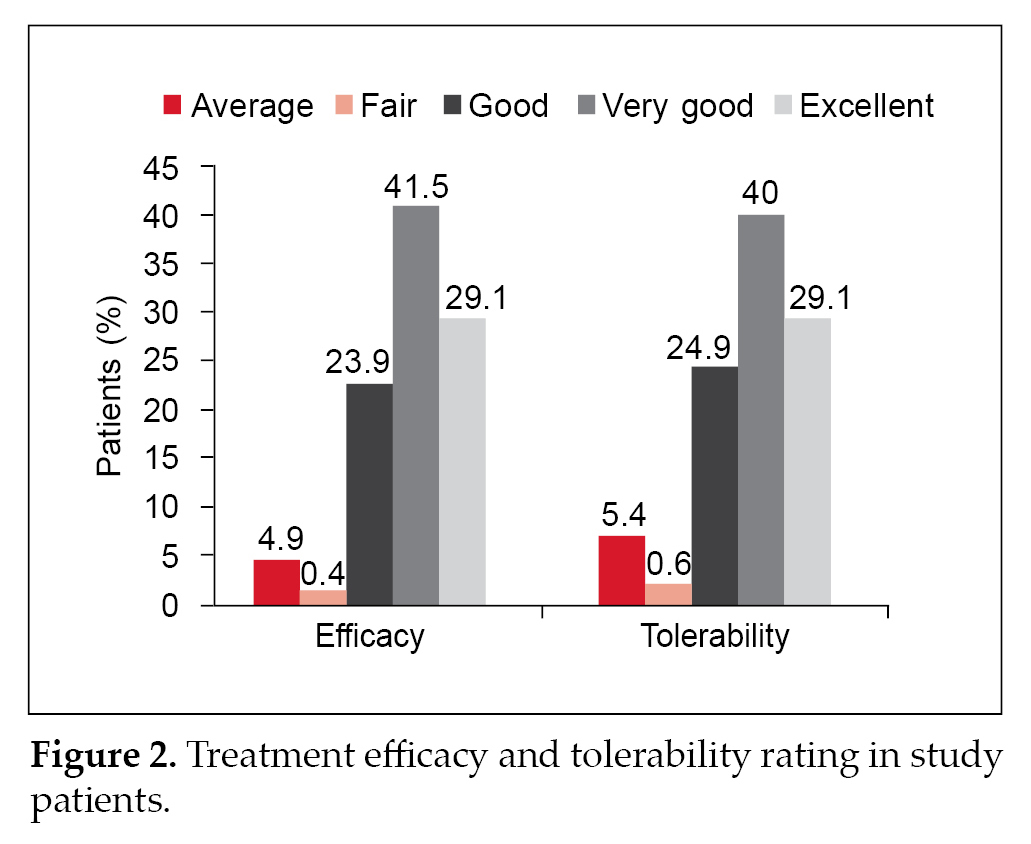
Physician evaluation of efficacy and tolerability were reported as good to excellent for 94.6% and 92.9% patients, respectively (Fig. 2).
DISCUSSION
India, with the second highest diabetes population in the world, faces several challenges in the management of this chronic disease. The high prevalence of comorbid conditions makes it tough for both the patient and the healthcare practitioner to appropriately manage the condition.8
The likelihood of macrovascular complications increases with hyperglycemia severity. While the available antidiabetic agents are effective for the management of hyperglycemia, most patients with T2DM are at a considerable risk for CVD.9
The present study explored the usage of glimepiride/metformin FDC in patients with T2DM who had CVD or were at risk of developing CVD. This case-based questionnaire survey also assessed the approach of HCPs across India regarding early use of glimepiride/metformin FDC in these patients.
About 18.1% patients in our study were reported to have developed CVD after diabetes was diagnosed, while 81.9% had CV issues at the time of diabetes diagnosis. Hypertension was the most common comorbidity in the study participants. This is in line with other studies conducted in Indian patients. A study by Pati and Schellevis also noted hypertension (62%) to be the most common comorbid condition in diabetes patients.8
In a real-world study by Sahay et al, 42.3% patients had hypertension.10 Similar results were noted by Prasanna Kumar et al.11
Other common comorbidities in our study included dyslipidemia (47.9%) and CAD (25.4%). Similar findings have been noted in other studies conducted in Indian T2DM patients, with other common comorbidities being dyslipidemia, CAD and neuropathy.10,11 One of the studies also noted retinopathy, nephropathy, peripheral vascular disease and diabetic foot as common comorbidities.12 These studies present a picture of the varied comorbid conditions seen in T2DM patients in India.
The co-existence of diabetes and comorbidities like hypertension can heighten the odds of micro- and macrovascular complications.13 Early combination therapy with glimepiride and metformin carries the advantage of a legacy effect, by means of early glycemic control and averting a negative glycemic memory tied to micro- and macrovascular complications.10
All patients included in this study received glimepiride/metformin FDC as first-line therapy. Besides, a large number of the patients received glimepiride/metformin FDC therapy soon after diagnosis (duration 1-5 years). This is in accordance with the recommendations of the American Diabetes Association (ADA), which recommends that early combination therapy has to be considered in some patients right at treatment initiation in order to delay treatment failure. In patients with HbA1c ≥1.5% above the target, dual combination therapy is needed.14 While the usual practice is to follow stepwise addition of antidiabetic medications to metformin to maintain A1c target, evidence now favors initial combination therapy in order to attain glycemic goals early.15
The CV risks associated with antidiabetic drugs have long been debated, especially those for SUs. However, there is ample data pointing to the CV-neutral effects of metformin and modern SUs now. Use of metformin is associated with a significant reduction in CV events and decrease in BP.16 It is associated with lower all-cause mortality, lower CV mortality and lower rates of MI and stroke. It also has potential favorable effects on some CV risk factors, such as plasma triglycerides, low-density lipoprotein (LDL), very low-density lipoprotein (VLDL) and high-density lipoprotein (HDL) levels.16 Metformin is also effective in reducing biomarkers of inflammation and endothelial dysfunction in T2DM patients and is tied to reduced CV risk, both with regard to mortality and incidence in patients with diabetes.17,18
The modern SU, glimepiride, is also associated with decreased CV risk, compared with other SUs.16 Modern SUs may not be linked to the unfavorable effects seen with conventional SUs. Glimepiride use is associated with decreased mortality rates in diabetes
patients with CAD when compared with glyburide and may be preferred in patients with underlying CAD.19 Based on recent reports from trials like ADVANCE, TOSCA.IT and CAROLINA, there seems to be no difference in CV risk between SUs and OHAs, like pioglitazone or linagliptin.20 In a recent comparison from the CAROLINA and CARMELINA trials, the investigators emphasized that the CV safety of glimepiride is re-assuring.21 An international clinical expert group also suggests that modern SUs are safe for use in T2DM patients with CV risk, MI or stroke.5 Glimepiride also has anti-atherosclerotic effect.22 The ADA and the Research Society for Study of Diabetes in India (RSSDI) also suggest that modern SUs, such as glimepiride, have a neutral CV risk profile.10
Combination therapy with metformin and SUs has also been reported to be as safe as metformin monotherapy
in terms of CV mortality and all-cause mortality.23 Ioacara et al noted a beneficial effect on all-cause mortality for SUs added to initial metformin monotherapy and also when metformin was added to initial SU monotherapy among T2DM patients.24 Metformin and glimepiride combination has also been associated with significant reduction in total cholesterol, triglyceride, LDL cholesterol and VLDL cholesterol levels, compared to metformin combined with sitagliptin or voglibose.25 These effects validate the use of a combination of glimepiride and metformin in T2DM patients who have CVD or are at risk of CVD, as seen in our study.
The use of this combination has become increasingly common in India. A recent real-world study in the Indian setting showed that glimepiride/metformin FDCs were commonly used in T2DM patients with comorbidities and diabetes complications. The authors concluded that glimepiride/metformin FDCs are appropriate for both early and long-standing diabetes.10 This is in line with our study, where all patients, across various age groups and diabetes duration, received glimepiride/metformin FDC as first-line therapy.
Around 7.9% patients in our study received insulin therapy along with glimepiride/metformin FDC treatment. Glimepiride/metformin combination therapy plus insulin has been reported to result in significant improvement in overall glycemic control.26 Prasanna Kumar and colleagues also corroborated the beneficial effects of glimepiride/metformin combination with insulin on glycemic control.11 Only 5.3% of the patients experienced hypoglycemia. Similar results were observed in a study by Unnikrishnan et al, where only 5.8% patients on glimepiride/metformin FDC therapy had a hypoglycemic event.12
Majority of the patients on glimepiride/metformin FDC therapy had BP within optimal limits (68.2%). Derosa and Sibilla have shown that antidiabetic medications might have a small, though significant, impact on BP in patients with T2DM.27 While metformin has beneficial effects on several CV risk factors and decreases cardiac events in overweight individuals with T2DM, newer SUs, such as glimepiride, are considered CV safe as they are more selective.27 Around 18.4% patients in this study had a change in weight and out of these patients, 59.2% had reduction in weight. Previous studies have; however, shown that combination therapy with glimepiride and metformin was associated with increase in weight.28 Improvements in glycemic parameters were noted in this study with the use of different strengths of glimepiride/metformin FDCs. A study by Pareek et al also showed that glimepiride/metformin combination led to improvement in metabolic control as determined by changes in HbA1c, FPG and PPG.29 Prasanna Kumar and colleagues also noted a significant reduction in HbA1c in patients treated with glimepiride/metformin combination along with insulin.11 The efficacy and tolerability were reported to be good to excellent for 92.79% and 92.2% of patients, respectively. The real-world study by Prasanna Kumar et al also noted similar findings with good to excellent tolerability irrespective of disease duration.11
This study includes data on key parameters such as HbA1c, FPG and PPG as well as on efficacy and tolerability with glimepiride/metformin FDC, which are valuable to interpret the effects of this combination in T2DM patients having CVD or at risk of developing CVD. The limitation of this study is its retrospective nature. There is a need to further validate these findings in large-scale prospective observational studies to understand the efficacy and safety of glimepiride/metformin FDCs in this patient population in the Indian setting.
CONCLUSION
This case-based questionnaire survey of the usage of glimepiride/metformin FDC in the Indian setting shows that various strengths of glimepiride/metformin FDCs are commonly prescribed in patients with T2DM who have CVD or have a risk of developing CVD, as a first-line therapy, with or without other OHAs. There was a significant improvement in glycemic parameters with weight loss and fewer hypoglycemia episodes with this combination, with the BP being within optimal limits for a majority of patients. It can be concluded that early initiation of this combination is widely prescribed to diabetes patients with CVD or those patients who are at risk of CVD.
Authorship
All authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgments
The authors would like to acknowledge Mr A Thamburaj and Ms Shashikala Borhade from USV Pvt. Ltd. For their assistance in the conduct of the project.
Contributors
KD Singh, Manish Gathoria, Naveen Sharma, Anil Javahirani, Praveer Sinha, Pankaj P Patil, Bhadresh S Shah, Nitin N Bote, Piyush Khera, Rajiv Rastogi, Kumar Shankha Poddar, Saumitra Ray, Kajal Ganguly, M Srinivas Rao, Johann Christopher, P Mallesh, RR Mantri, Gaurav Minocha, Niroj Kumar Mishra, Chandrakanta Mishra, Thomas John, Manoj Ravi, Vikram B Kolhari, Mahendra Prasad Samal, Rohan V Ainchawar, Ashutosh Sahu, Sandip Fulpagare, Amit Sharma, Somnath Mukhopadhay, Arindam Pande, Pankaj Sarkar, Allam Vasanth Kumar, Kailash Pabba, Sumeet Sinha, Mohammed Wasif Azam, G Krishnamurthy, Yerra Shiva Kumar, KRKS Raju, Pawan Poddar, VS Srinath, PA Jiwani, KS Sadananda, Amit Kumar Jain, Subhash Chander Manchanda, Ram Narain Kalra, Ripen Kumar Gupta, M Nanda Kumaran, Rohit Shrivastava, Gajjala Rama Krishna Reddy, Rajesh Wagh, Chetan
P Shah, Mahesh Chandra, Anurag Mehrotra, Pulala Chandra Sekhar, Jubili P Mathew, EM Arunachalam, Nitin Modi, Idris Ahmed Khan, Shashank Dixit, Naga Mallesh, Arun K Kedia, Brijesh Agrawal, S Ramanathan, Babuchakravorthy K, Navneet Jaipuriar, Somnath Mallakmir, Mohd Ahmad, Kaushik Naha Biswas, Lakshmana S Sridhar, Kumar Rajeev, Mayur N Jain, Parnesh Arora, Rajendra K Nayak, Dhiren R Shah, Paramartha Bhattacharya, Soupayan Dutta, Ranjan Kumar Sharma, Durga Prasad Chakraborty, Goutam Sarkar, Arun Srinivas, Arshad M, P Sivasamy, D Chakkravarthi, J Jegadeesh, Kaushik Chaudhuri, Animesh Das, Ashwani Mehta, Jitendra Pal Singh Sawhney
Funding
The project has been funded by USV Pvt. Ltd.
Conflict of Interest
There are no conflicts of interest to declare.
REFERENCES
- Diabetes and cardiovascular diseases. IDF Diabetes Atlas. 9th Edition, 2019.
- Gerstein HC. Diabetes: Dysglycaemia as a cause of cardiovascular outcomes. Nat Rev Endocrinol. 2015;11(9):508-10.
- Baker C, Retzik-Stahr C, Singh V, Plomondon R,
Anderson V, Rasouli N. Should metformin remain the first-line therapy for treatment of type 2 diabetes? Ther Adv Endocrinol Metab. 2021;12:2042018820980225.
- Holman RR, Paul SK, Bethel A, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-89.
- Kalra S, Das AK, Baruah MP, Unnikrishnan AG, Dasgupta A, Shah P, et al. Glucocrinology of modern sulfonylureas: clinical evidence and practice-based opinion from an International Expert Group. Diabetes Ther. 2019;10(5):1577-93.
- Kalra S, Ghosh S, Das AK, Nair T, Bajaj S, Priya G, et al. Unravelling the utility of modern sulfonylureas from cardiovascular outcome trials and landmark trials: expert opinion from an international panel. Indian Heart J. 2020;72(1):7-13.
- Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, et al; CAROLINA Investigators. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322(12):1155-66.
- Pati S, Schellevis FG. Prevalence and pattern of co morbidity among type 2 diabetics attending urban primary healthcare centers at Bhubaneswar (India). PLoS One. 2017;12(8):e0181661.
- Paneni F, Lüscher TF. Cardiovascular protection in the treatment of type 2 diabetes: a review of clinical trial results across drug classes. Am J Cardiol. 2017;120(1S):S17-S27.
- Sahay RK, Mittal V, Gopal GR, Kota S, Goyal G, Abhyankar M, et al. Glimepiride and metformin combinations in diabetes comorbidities and complications: real-world evidence. Cureus. 2020;12(9):e10700.
- Prasanna Kumar KM, Seshadri K, Aravind SR, Deb P, Modi KD, Gopal RA, et al. Real-world observational study of glimepiride and metformin fixed-dose combination along with insulin in the management of type 2 diabetes mellitus: Indian experience. Cureus. 2021;13(1):e13020.
- Unnikrishnan AG, Pandit K, George J, Venkataraman S,
Abhyankar M. Clinical utilization pattern of multiple strengths of glimepiride and metformin fixed dose combinations in Indian type 2 diabetes patients. J Assoc Physicians India. 2020;68(7):57-61.
- Sharma A, Mittal S, Aggarwal R, Chauhan MK. Diabetes and cardiovascular disease: inter-relation of risk factors and treatment. Future J Pharm Sci. 2020;6:130.
- American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45
(Suppl 1):S125-43.
- Phung OJ, Sobieraj DM, Engel SS, Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab. 2014;16(5):410-7.
- Azimova K, San Juan Z, Mukherjee D. Cardiovascular safety profile of currently available diabetic drugs. Ochsner J. 2014;14(4):616-32.
- Lund SS, Tarnow L, Stehouwer CD, Schalkwijk CG, Teerlink T, Gram J, et al. Impact of metformin versus repaglinide on non-glycaemic cardiovascular risk markers related to inflammation and endothelial dysfunction in non-obese patients with type 2 diabetes. Eur J Endocrinol. 2008;158(5):631-41.
- Zhang K, Yang W, Dai H, Deng Z. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: results from meta-analysis. Diabetes Res Clin Pract. 2020;160:108001.
- Pantalone KM, Kattan MW, Yu C, Yu C, Wells BJ, Arrigain S,
et al. The risk of overall mortality in patients with type 2
diabetes receiving glipizide, glyburide, or glimepiride monotherapy: a retrospective analysis. Diabetes Care. 2010;33(6):1224-9.
- Leiter LA. Latest evidence on sulfonylureas: what’s new? Diabetes Ther. 2020;11(Suppl 1):15-22.
- Ghosh S, Mukhopadhyay P, Pandey P, Chatterjee P,
Pandit K. Cardiovascular safety of glimepiride: an indirect comparison from CAROLINA and CARMELINA. Diab Vasc Dis Res. 2020;17(6):1479164120973653.
- Koshiba K, Nomura M, Nakaya Y, Ito S. Efficacy of glimepiride on insulin resistance, adipocytokines, and atherosclerosis. J Med Invest. 2006;53(1-2):87-94.
- Sillars B, Davis WA, Hirsch IB, Davis TM. Sulphonylurea-metformin combination therapy, cardiovascular disease and all-cause mortality: the Fremantle Diabetes Study. Diabetes Obes Metab. 2010;12(9):757-65.
- Ioacara S, Guja C, Reghina A, Martin S, Sirbu A, Fica S. All-cause and cardiovascular mortality associated with sulphonylurea and metformin therapy in type 2 diabetes. Endocr Res. 2018;43(2):97-105.
- Kala P, Rani RJ. Hypolipidemic effect of sitagliptin, voglibose and glimepiride in combination with metformin in patients with type 2 diabetes mellitus at a tertiary care teaching hospital: a comparative study. Int J Basic Clin Pharmacol. 2019;8(6):1268-72.
- Park CY, Kang JG, Chon S, Noh J, Oh SJ, Lee CB, et al. Comparison between the therapeutic effect of metformin, glimepiride and their combination as an add-on treatment to insulin glargine in uncontrolled patients with type 2 diabetes. PLoS One. 2014;9(3):e87799.
- Derosa G, Sibilla S. Optimizing combination treatment in the management of type 2 diabetes. Vasc Health Risk Manag. 2007;3(5):665-71.
- Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al; LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes. The LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care. 2009;32(1):84-90.
- Pareek A, Chandurkar NB, Salkar HR, Borkar MS, Tiwari D.
Evaluation of efficacy and tolerability of glimepiride and metformin combination: a multicentric study in patients with type-2 diabetes mellitus, uncontrolled on monotherapy with sulfonylurea or metformin. Am J Ther. 2013;20(1):41-7.