Abstract
Background: Type 2 diabetes mellitus (T2DM) poses a major public health burden. The present case-based questionnaire survey evaluated the treatment pattern and clinical experience of healthcare professionals (HCPs) in prescribing glimepiride/metformin fixed-dose combination (FDC) with insulin, with or without other oral hypoglycemic agents (OHAs), to patients with T2DM in the Indian setting. Material and methods: A retrospective, multicenter, observational, case-based questionnaire survey was conducted at several healthcare centers in India with the help of medical records of patients having T2DM, who were prescribed different strengths of glimepiride/metformin FDC. Data was collected from the patients’ medical records and were analyzed using statistical tests. Results: A total of 1,013 patients with T2DM were included in this study. The mean (± standard deviation [SD]) age of patients was 53.5 ± 13.9 years. Mean duration of diabetes was 6.3 ± 4.8 years. About 70.1% of the patients received glimepiride/metformin FDC as first-line therapy and 29.9% received it as second-line therapy. Around 66.3% of the patients in first-line glimepiride/metformin FDC group received insulin once a day, and the proportion increased to 86.8% of the patients in second-line therapy group. Other OHAs were used in 754 (74.4%) patients. About 18.2% (n = 185) patients reported change in weight, with a slightly larger number of patients having reduction in weight. There was considerable reduction in HbA1c, FPG and PPG in patients receiving glimepiride/metformin FDC with insulin, irrespective of OHA use. Efficacy and tolerability were reported as good to excellent for 96.2% and 94.8% patients, respectively. Conclusion: This case-based questionnaire survey shows the usage pattern of various strengths of glimepiride/metformin FDC with insulin
and the HCPs’ practice approach regarding early initiation of this combination in Indian patients with T2DM.
Keywords: Type 2 diabetes mellitus, glimepiride/metformin combination, combination therapy, insulin
The global public health crisis of type 2 diabetes mellitus (T2DM) has already attained the status of a pandemic, and has seen a shift from the developed world to the developing nations of Asia, Africa and Latin America.1 India has the second largest number of adults with diabetes globally, and is expected to retain the spot even in 2045.2
Type 2 diabetes is a metabolic disorder which occurs as a result of either deficient insulin secretion, pancreatic β-cell damage or insulin resistance. The noninsulin pharmacological management of type 2 diabetes involves several different drug classes, including biguanides, insulin secretagogues (sulfonylureas [SUs] and mitiglinides), insulin sensitizers (peroxisome proliferator-activated receptor [PPAR] agonists), alpha-glucosidase inhibitors (AGIs), incretin mimetics (glucagon-like peptide 1 [GLP-1] agonists and dipeptidyl peptidase-4 [DPP-4] inhibitors), amylin antagonists and sodium-glucose cotransporter-2 (SGLT2) inhibitors.3
Metformin, a biguanide, is the choice of initial pharmacologic agent for the treatment of type 2 diabetes. Biguanides diminish intestinal glucose absorption and limit hepatic glucose production as well as output as they decrease gluconeogenesis and stimulate glycolysis. This drug class does not cause hypoglycemia or lead to weight gain, has antihypertriglyceridemic effect and can reduce the risk of cardiovascular (CV) events.3,4
Considering the progressive nature of T2DM, it may not be possible to maintain the glycemic targets with monotherapy beyond a few years. Combination therapy becomes necessary in order to achieve glycemic control and delay β-cell deterioration. While it is recommended to follow stepwise addition of drugs to metformin, initial combination therapy may also be considered to quickly attain glycemic goals in some patients.3,4
Sulfonylureas are the agents which have a large body of evidence, experience and outcome data to support their role in managing patients with diabetes. The World Health Organization (WHO) recommends using an SU as second-line treatment in patients who fail to achieve glycemic control with metformin alone or have contraindications to metformin therapy, especially in resource-limited settings where a large number of patients have to pay for their treatment out of their own pocket.5 Modern SUs, such as glimepiride, have an established safety and efficacy profile in T2DM patients. SUs can safely be used in combination with all classes of oral hypoglycemic agents (OHAs), except glinides.6
Additionally, modern SUs are cardio-safe. The CV safety of SUs was established by the CAROLINA trial, the first cardiovascular outcome trial (CVOT) that compared glimepiride with DPP-4 inhibitor linagliptin and reported no difference in CV risk between the groups.7 The risk of hypoglycemia is also reduced with the use of modern SUs, such as glimepiride, and they have weight neutral effects, when compared with conventional SUs.6 SUs can therefore be used across the diabetes continuum right from an early stage as monotherapy added to lifestyle measures, as dual or triple therapy, or as add-on to basal insulin.8
Oral antidiabetic drugs (OADs) sometimes fail, or are inadequate, to achieve the target glycemic control and maintain it. This OAD failure or inadequacy necessitates the use of insulin therapy.9 The rationale for insulin and OAD combination can be appreciated after understanding the pathophysiology of T2DM and the action of the oral agents. Patients with T2DM are insulin-deficient as well as insulin-resistant, thus requiring high doses of exogenous insulin. Secondly, peripheral insulin delivery results in hyperinsulinemia, which eventually contributes to late complications. SUs act by stimulating insulin release into the portal vein and have a role in enhancing peripheral insulin action. Meanwhile, metformin enhances glucose metabolism and insulin sensitivity and decreases the amount of insulin required.10 OADs alone may not be able to achieve and maintain glycemic control on account of a deterioration in β-cell function. Hence, the need for exogenous insulin. Combination therapy with OADs and insulin can yield excellent glycemic control, reduce insulin dosages and certain combinations can even check the weight gain seen with insulin therapy.11
Sustained glycemic control may not be achieved in many patients with insulin combination with non-SU drugs.12 A combination of insulin and SU has rather been reported to be more effective than insulin alone in the treatment of diabetes patients with better glucose profiles and reduced insulin requirement.10 For instance, addition of glimepiride to insulin treatment has been shown to result in greater improvement in glycemic control with a significantly smaller daily insulin dose.12 Use of glimepiride/metformin combination plus insulin has also been reported to yield greater reduction in blood glucose levels than glimepiride plus insulin.13 In a study by Prasanna Kumar et al, glimepiride/metformin combination with insulin led to reduction in glycated hemoglobin (HbA1c) in T2DM patients with a mean change of 1.33%.14
Considering the benefits of glimepiride and metformin in combination with exogenous insulin in the management of T2DM, there is a need for physician opinion on glimepiride/metformin FDC along with insulin amongst Indian T2DM patients.
A case-based questionnaire survey was conducted to evaluate the demography, treatment pattern, including duration and various dosages of glimepiride/metformin FDC used with insulin in the management of T2DM.
MATERIAL AND METHODS
Study Design
This was a retrospective, multicenter, observational, case-based questionnaire survey. It was conducted with 147 healthcare professionals (HCPs) across different centers in India from July 2020 through May 2021. The study protocol was designed in accordance with the principles of the Declaration of Helsinki.
Study Population
Patients of both sexes, aged above 18 years, diagnosed with T2DM who received a glimepiride/metformin FDC in any strength along with insulin were recruited in the study.
Data Collection
A case report format was developed to determine the pattern of use of different strengths of glimepiride/metformin FDCs with insulin with or without other OHAs in diabetes patients. The questionnaire was sent to 147 HCPs across India via an online portal. Questions regarding demographic characteristics, such as age, sex, body mass index (BMI) and medical history; duration of diabetes; comorbidities; biochemical measures, such as fasting plasma glucose (FPG), postprandial plasma glucose (PPG) and HbA1c levels; antidiabetic drugs used; antidiabetic drug up-titrations and
down-titrations; weight change; hypoglycemic episodes and other adverse events during treatment, were included in the questionnaire. An online portal was developed where the HCPs filled in the information. A descriptive analysis was performed with the data provided on the portal.
Statistical Analysis
Descriptive statistical analyses, including mean and standard deviation (SD) for continuous variables and count and percentage for categorical variables, have been performed. Fisher’s exact test was used for two categorical variables with two categories. For categorical variables with more than two categories, Chi-square test was used. All the reported p-values are two-sided and p-values <0.05 is considered to indicate statistical significance. All data entries and statistical analyses were performed using SPSS® Version 23.0 software.
RESULTS
A total of 1,013 patients with T2DM were included in this retrospective observational questionnaire-based analysis (612 male and 401 female). The mean (±SD) age of patients was 53.5 ± 13.9 years. Table 1 summarizes the distribution of patients according to age. The distribution of patients in different age groups based on duration of diabetes is shown in Table 2.
A total of 121 patients (11.9%) were in the normal BMI category (18.5-22.9 kg/m2), 147 (14.5%) were overweight (23-24.9 kg/m2) and 745 (73.5%) were obese (>25 kg/m2). Mean duration of diabetes was 6.3 ± 4.8 years. About 45.1% of patients had diabetes duration of 1 to 5 years (n = 457), followed by 6 to 10 years (33.1%, n = 335). The least number of patients had diabetes duration of >20 years (n = 11).
About 70.1% of the patients received glimepiride/metformin FDC as first-line therapy and 29.9% received it as second-line therapy.
The most commonly prescribed glimepiride/metformin FDC regimen was glimepiride 0.5 mg + metformin 500 mg. Table 3 summarizes the dosage regimens used in study participants.
Dose titration was done in only 160 (15.1%) patients. Out of these, up-titration was done in 84 patients and down-titration was done in 61 patients (data was not available for some patients).
Around 66.3% of the patients in first-line glimepiride/metformin therapy group received insulin once a day. This increased to 86.8% of the patients in second-line therapy group (Table 4). Overall, 754 (74.4%) patients also received another OHA. Among these patients, 295 (39.1%) received SGLT2 inhibitors, 89 (11.8%) received thiazolidinediones, 449 (59.5%) received DPP-4
inhibitors, 115 (15.2%) received AGIs and 10 (1.3%) received GLP-1 agonists.
Hypoglycemia at 6 months was noted in 34.5% patients. There were no other major adverse events. About 18.2% (n = 185) patients reported change in weight, with a slightly larger number of patients (n = 96) having reduction in weight.
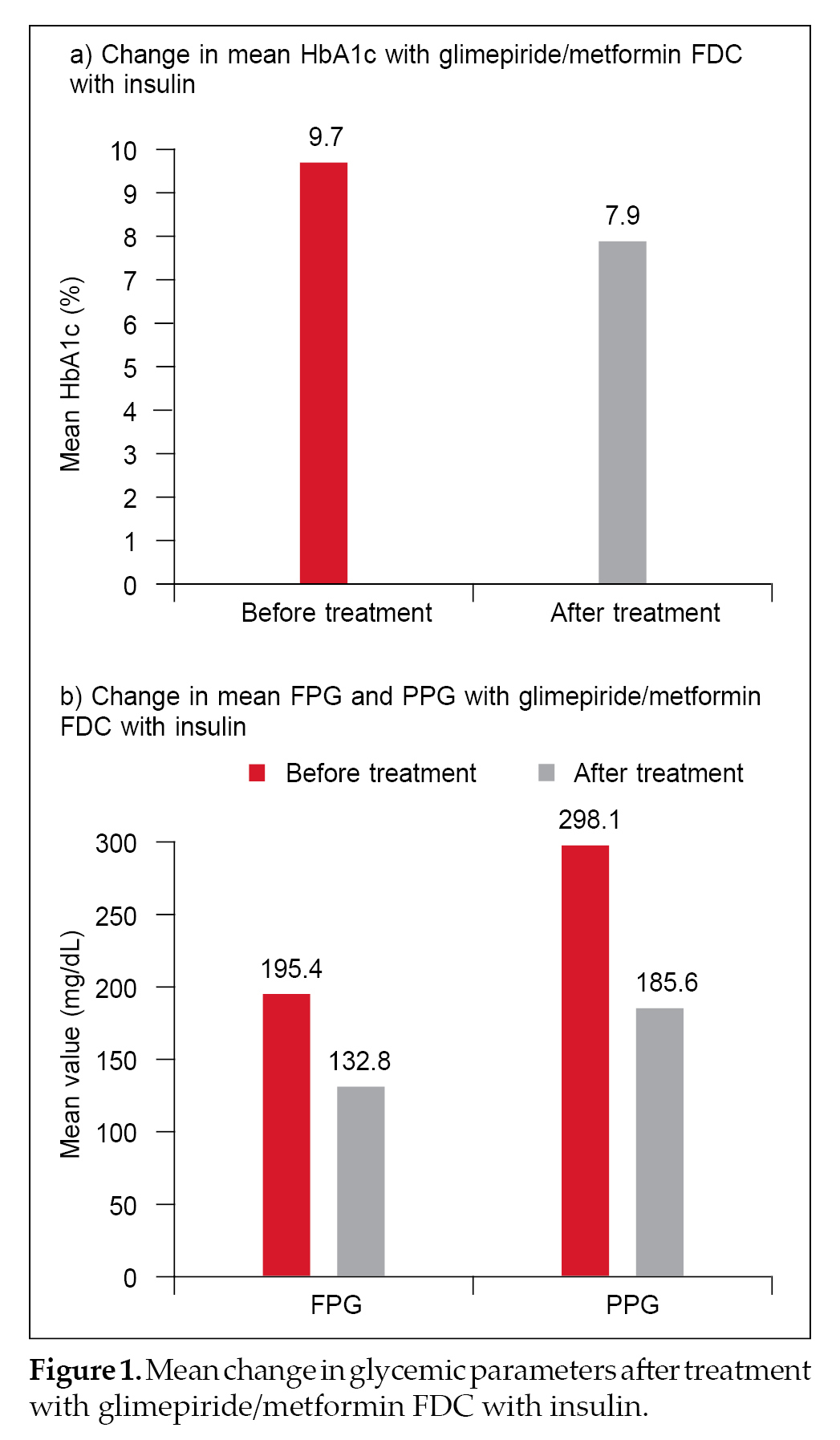
A total of 624 (61.6%), 502 (49.6%) and 490 (48.3%) patients had a reduction in HbA1c, FPG and PPG. Mean HbA1c values decreased after treatment with glimepiride/metformin FDC plus insulin from
9.7% ± 1.3% to 7.9% ± 4.2%. Mean FPG and PPG were also reduced post-treatment from 195.4 ± 42.8 mg/dL to
132.8 ± 34.3 mg/dL and from 298.1 ± 59.5 mg/dL to 185.6 ± 39.3 mg/dL, respectively (Fig. 1 a and b). The percentage reduction in HbA1c, FPG and PPG was 18.55%, 32.04% and 37.74%, respectively.
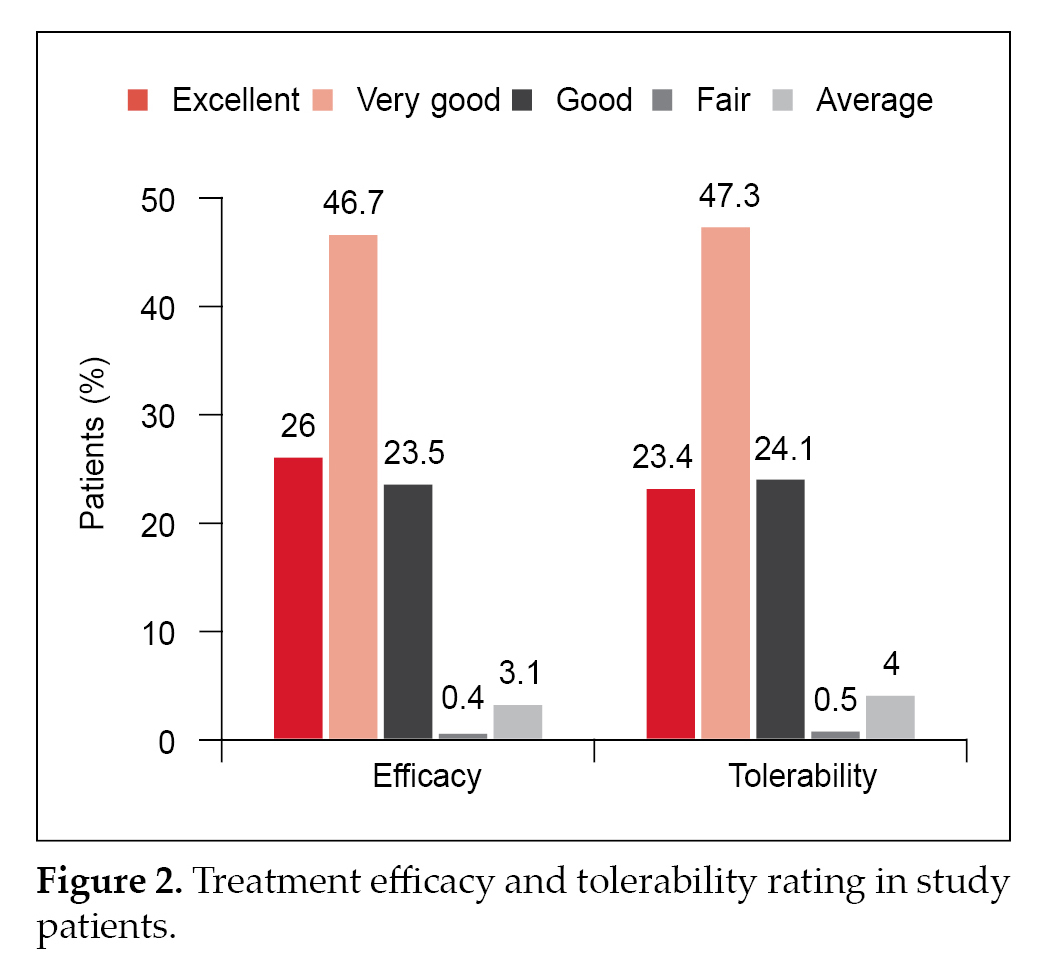
Physician evaluation of efficacy and tolerability were reported as good to excellent for 96.2% and 94.8% patients, respectively (Fig. 2).
|
Table 1. Distribution of Patients According to Age
|
|
Age group
|
No. of patients
|
Percentage (%)
|
|
18-25
|
31
|
3.1
|
|
26-45
|
258
|
25.5
|
|
46-59
|
372
|
36.7
|
|
60-75
|
299
|
29.5
|
|
>75
|
53
|
5.2
|
|
Total
|
1,013
|
100.0
|
|
Table 2. Distribution of Patients in Different Age Groups Based on Duration of Diabetes
|
|
Age group (years)
|
Newly diagnosed
|
Diabetes duration (years)
|
|
1-5
|
6-10
|
11-15
|
16-20
|
Over 20
|
|
18-25
|
22 (53.7)
|
5 (1.1)
|
4 (1.2)
|
0 (0)
|
0 (0)
|
0 (0)
|
|
26-45
|
6 (14.6)
|
173 (37.9)
|
71 (21.2)
|
8 (6.3)
|
0 (0)
|
0 (0)
|
|
46-59
|
6 (14.6)
|
128 (28)
|
175 (52.2)
|
50 (39.4)
|
11 (26.2)
|
2 (18.2)
|
|
60-75
|
5 (12.2)
|
116 (25.4)
|
80 (23.9)
|
65 (51.2)
|
26 (61.9)
|
7 (63.6)
|
|
>75
|
2 (4.9)
|
35 (7.7)
|
5 (1.5)
|
4 (3.1)
|
5 (11.9)
|
2 (18.2)
|
|
Total
|
41
|
457
|
335
|
127
|
42
|
11
|
The values are described as n (%).
|
Table 3. Different Strengths of Glimepiride/Metformin FDC Prescribed to Study Participants
|
|
Glimepiride/Metformin FDC regimen
|
No. of patients
|
Percentage (%)
|
|
Glimepiride 0.5 mg + Metformin 1000 mg
|
8
|
0.8
|
|
Glimepiride 1 mg + Metformin 1000 mg
|
66
|
6.5
|
|
Glimepiride 2 mg + Metformin 1000 mg
|
83
|
8.2
|
|
Glimepiride 3 mg + Metformin 1000 mg
|
25
|
2.5
|
|
Glimepiride 4 mg + Metformin 1000 mg
|
38
|
3.8
|
|
Glimepiride 0.5 mg + Metformin 500 mg
|
299
|
29.5
|
|
Glimepiride 1 mg + Metformin 500 mg
|
189
|
18.7
|
|
Glimepiride 2 mg + Metformin 500 mg
|
203
|
20.0
|
|
Glimepiride 1 mg + Metformin 850 mg
|
29
|
2.9
|
|
Glimepiride 2 mg + Metformin 850 mg
|
28
|
2.8
|
|
Glimepiride 3 mg + Metformin 850 mg
|
45
|
4.4
|
|
Total
|
1,013
|
100.0
|
|
Table 4. Insulin Dosage Regimen Used in Patients on Glimepiride/Metformin FDC Therapy
|
|
Insulin
|
Glimepiride/Metformin FDC therapy
|
|
First-line therapy
|
Second-line therapy
|
|
BD
|
239 (33.7)
|
40 (13.2)
|
|
OD
|
471 (66.3)
|
263 (86.8)
|
|
Total
|
710 (70)
|
303 (29.9)
|
The values are described as n (%).
BD: Twice a day; OD: Once a day.
DISCUSSION
The present case-based questionnaire survey evaluated the usage of glimepiride/metformin FDC with insulin, with or without other OHAs, in patients with T2DM.
It looked at the approach of HCPs across India regarding the use of glimepiride/metformin FDC along with insulin in people with diabetes.
Around 36.7% of the patients were in the 46 to 59 years age group. In this particular age group, more
than half of the patients (52.2%, n = 175) had a diabetes duration of 6 to 10 years. A vast majority of patients (70.1%) received glimepiride/metformin FDC as first-line therapy. This is in line with the American Diabetes Association (ADA) recommendation that early combination therapy may be needed in some patients to delay treatment failure.4 The conventional stepwise approach may result in a delay in achieving the glycemic goals. Moreover, the up-titration of monotherapy may be associated with untoward effects. Thus, early combination therapy seems to be a judicious approach where submaximal doses of the antidiabetic agents can be combined to yield better glycemic control with minimal side effects.15
Glimepiride/metformin combination is a time-tested treatment regimen in T2DM management. This combination has been reported to be more effective than metformin up-titration to achieve glycemic control in patients uncontrolled on metformin low-dose monotherapy.16 Moreover, within the SU class, glimepiride has been found to be a better alternative to other SUs, in combination with metformin. A study by González-Ortiz and colleagues found glimepiride/metformin combination to be more effective compared to glibenclamide/metformin combination to attain glycemic control and was associated with less hypoglycemic events.17 In the Indian setting as well, this is a widely used OAD combination.18 Fixed-dose combinations of glimepiride and metformin are extensively used in India, considering their availability in a wide range of strengths, and this is associated with an ease of titration as well.19 An added advantage in the Indian setting is that this combination is a cost-effective treatment approach.18 Sahay and colleagues, in their real-world study in T2DM patients, noted that glimepiride/metformin FDCs are commonly used in those with comorbidities and diabetes complications. The investigators stated that these combinations are well suited for both early and long-term diabetes.18 Unnikrishnan et al noted in a case-based questionnaire survey that glimepiride/metformin FDC has potential benefits in patients with T2DM, regardless of age, duration of diabetes, BMI, diabetes complications, as well as the use of concomitant medications, like insulin.19 A study conducted in Nepal also noted glimepiride/metformin low-dose combination (glimepiride 0.5 mg/metformin 500 mg) to be effective in T2DM patients, across an age range of 23 to 85 years, for glycemic control. There was an average 26% reduction in FPG and PPG in the patients.20
These results were replicated in our study, where the benefits of glimepiride/metformin FDC were evident in T2DM patients across age groups, BMI categories, diabetes duration and use of other OHAs. Use of glimepiride/metformin FDC with insulin has also been reported to be efficacious in attaining glycemic control. Yu et al reported that glimepiride/metformin FDC along with insulin is associated with reduction in blood glucose levels in T2DM patients and is a relatively safe option.13 Park and colleagues also noted in their study that use of glimepiride/metformin with insulin was associated with significantly greater improvement in glycemic control vs. treatment with insulin and glimepiride or insulin and metformin.21
In an Indian study, glimepiride/metformin FDC usage with insulin was shown not to increase the risk of hypoglycemia and weight gain. The study showed that different strengths of glimepiride/metformin FDCs are used with insulin in diabetes patients, without any increased risk of adverse events.19 Our study had similar findings where a higher number of patients receiving glimepiride/metformin FDC with insulin reported weight reduction and there were no increased risk of adverse events.
A real-world study by Prasanna Kumar et al, conducted in India, noted good HbA1c lowering with glimepiride/metformin combination with insulin and the most frequently used glimepiride/metformin regimen in this study was glimepiride 2 mg/metformin 500 mg.14 In our study as well, there was considerable reduction in HbA1c, FPG and PPG levels in patients receiving glimepiride/metformin FDC with insulin, with the most frequently used regimen being glimepiride 0.5 mg/metformin 500 mg. Physician evaluation of efficacy and tolerability were reported as good to excellent for 96.2% and 94.8% patients, respectively. This is similar to the results of the study by Prasanna Kumar et al, where physician global evaluation of efficacy and tolerability revealed that a vast majority of patients were on good to excellent (97.3% and 96.6%).14
This study has presented data on key glycemic parameters such as HbA1c, FPG and PPG and demonstrated the efficacy and tolerability of glimepiride/metformin FDC with insulin, with or without other OHA use, in T2DM patients. These results provide valuable insights into the effects of this regimen in these patients.
However, the retrospective nature of this study is one of its limitations. Additionally, the glycemic control achieved in this study could not be correlated with different glimepiride/metformin FDCs prescribed since patients also received other OADs. Furthermore, it could not be determined as to which individual therapeutic agent led to adverse events, if any, because combination therapy was given. The results should be further validated in large-scale prospective studies in order to assess the efficacy and safety of glimepiride and metformin combination with insulin in Indian patients with type 2 diabetes.
CONCLUSION
This case-based questionnaire survey of the usage of glimepiride/metformin FDC with insulin, with or without other OHAs, in the Indian setting revealed that different strengths of glimepiride/metformin FDCs are commonly prescribed along with insulin in T2DM patients. Majority of the patients received once daily insulin dose with glimepiride/metformin FDC. A large proportion of patients attained reduction in key glycemic parameters with this regimen.
Glimepiride/metformin FDC with insulin is commonly used in diabetes patients in India and this treatment approach has a favorable efficacy and tolerability profile.
Authorship
All authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgments
The authors would like to acknowledge Mr A Thamburaj and Ms Shashikala Borhade from USV Pvt. Ltd. For their assistance in the conduct of the project.
Contributors
MA Karmur, Vijay Shantilal Mehta, Piyush Dhirjilal Patel, Vinay Patel, Nitin J Patel, PV Swami, Sangamesh Ravi Chavanda, Sunil R Karande, Vinaykumar G Bhopatkar, Ravindra R Gundeli, Mahendra N Sonwane, Anand Shivnarayan Malani, Rahul G Ware, Aheson Shaikh, Kishor Pargaonkar, Anand M Londhe (Patil), Rajendra Purushottam Gondhali, Sharad Pandurang Dhawde, Atul M Shinde, Gajanan Ragvendra Deshpande, Shripad Vithalrao Dhanorkar, Satish Guthe, Harshal Ramesh Kakade, HB Kathuria, Pradeep Tilekar, Asif Bhojani, Kinaz S Ansari, Sachin Jaybhaye, Pradeep G Talwalkar, Avinash Shrikrishna Choudhary, Pravin Dinkar Supe, Vikas Namdeo Desale, Shailendra Pratapsingh Mahale, Kavita Krishna, Kothari Ramprakash Ramvallabh, Birju S Mori, Nilesh M Detroja, Bhavesh N Patel, Amit Agrawal, Shabbir S Gadi, Chetan Ramgopalgi Sarda, Tanaji V Chandawar, Rushikesh Maheshwari, Rishikesh Mehetre, Baliram Prabhakarrao Bagal, Jeet Singh, Amol K Pathak, Jyoti Gayal, Harsh Dilip Shah, Sonali A Patange, Kapil Omprakash Rathi, Shubhashree Patil, Abhay Raut, Ajay V Kaduskar, Gira Soni, Prasad Game, Sagar D Rakecha, Makarand Premlal Patel, Vinay Vasant Thakar, Yogesh Varge, Swapnil Wath, Ravindra Choudhari, Rajkumar Bhaskar Patil, Chudaman Pundlik Patil, Hemkant M Patil, Pradeep Kiran Jain, Sameer Chandratre, Varsha Kulkarni, Pravin Sudhakar More, Shubhada A Dharmadhikari, Malvika Amol Zantye, Kalpana S Mehta, Sonali Bhojane, Sachin Vasant Yadav, Ashish R Tapadia, Pravishal D Adling, Nitin Gade, Sanjay G Chaudhary, Vishwanath Balaji Parsewar, Mikal Y Khanna, Sahil N Fulara, Nirav Tanna, Abdul Hannan, Vinay Kashinath Parvatkar, Rajiv L Joshi, Mukesh Budhwani, Ronak Shah, Bharat R Sharma, Kirit N Parikh, Mitesh M Sutaria, Roopal Panchani, Vanraj M Hada, Hitesh Patel, Sachin K Katarkar, Shardul Kothary, Kapil Patwardhan, Shailesh Palekar, Samrat D Shah, Manish Vanage, Mukul Thakur, Prakash S Khetani, Aniruddha Tongaonkar, Abhishek M Karmalkar, Asmit Vaidya, SK Pal, Fahad I Merchant, GT Rane, Javed Shaikh, Pankaj Desai, Darshan V Pandya, Bhagwat J Mane, Ram A Shinde, Seema A Bagri, Gaurav Beswal, Aqeel Huseini Malbari, Pradip P Shah, Mahesh Gandhi, Amit Chandrakant Botre, KC Potdar, Manish Sachdev, Behram S Pardiwala, Azizullah Khan, Sujata N Mehta, Rohan Sequeria, Nitin Kesarkar, Sayiprasad, Ashok H Sancheti, Chandrashekar Patil, Snehal T Sanghavi, Prashant Kawadkar, SK Agarwal, Amol A Manerkar, Ashish Sarawate, Sharad Kamble, Dimple Vinubhai Patel, Pinakin Soni, Satish Naik, Mihir S Raut, Avanti Pardeshi, Kunjal Kamath, Preeti Gholap, Tejal Dravid, Girish Date, Avinash Mukadam, Sudhir Seth, Jyoti Vyas, Sandesh Maruti Galande
Funding
The project has been funded by USV Pvt. Ltd.
Conflict of Interest
There are no conflicts of interest to declare.
REFERENCES
- Unnikrishnan R, Pradeepa R, Joshi SR, Mohan V. Type 2 diabetes: demystifying the global epidemic. Diabetes. 2017;66(6):1432-42.
- Global picture (Chapter 3). IDF Diabetes Atlas. 9th Edition, 2019.
- Padhi S, Nayak AK, Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharmacother. 2020;131:110708.
- American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Suppl 1): S111-24.
- Mohan V, Khunti K, Chan SP, Filho FF, Tran NQ, Ramaiya K, et al. Management of type 2 diabetes in developing countries: balancing optimal glycaemic control and outcomes with affordability and accessibility to treatment. Diabetes Ther. 2020;11(1):15-35.
- Kalra S, Bahendeka S, Sahay R, Ghosh S, Md F, Orabi A, et al. Consensus recommendations on sulfonylurea and sulfonylurea combinations in the management of type 2 diabetes mellitus - International Task Force. Indian J Endocrinol Metab. 2018;22(1):132-57.
- Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, et al; CAROLINA Investigators. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322(12):1155-66.
- Scheen AJ. Sulphonylureas in the management of type 2
diabetes: to be or not to be? Diab Epidemiol Manag. 2021;1:100002.
- Jindal S, Kalra S. Developing a definition for oral antidiabetic drug (OAD) failure. J Pak Med Assoc. 2020;70(3):547-51.
- Scheen AJ, Castillo MJ, Lefèbvre PJ. Combination of oral antidiabetic drugs and insulin in the treatment of non-insulin-dependent diabetes. Acta Clin Belg. 1993;48(4):259-68.
- Buse J. Combining insulin and oral agents. Am J Med. 2000;108 Suppl 6a:23S-32S.
- Li CJ, Zhang JY, Yu DM, Zhang QM. Adding glimepiride to current insulin therapy increases high-molecular weight adiponectin levels to improve glycemic control in poorly controlled type 2 diabetes. Diabetol Metab Syndr. 2014;6(1):41.
- Yu HM, Kim SJ, Chun SW, Park KY, Lim DM, Lee JM, et al. A comparison study on efficacy, insulin sensitivity and safety of glimepiride/metformin fixed dose combination versus glimepiride single therapy on type 2 diabetes mellitus patients with basal insulin therapy. Diabetes Res Clin Pract. 2019;155:107796.
- Prasanna Kumar KM, Seshadri K, Aravind SR, Deb P, Modi KD, Gopal RA, et al. Real-world observational study of glimepiride and metformin fixed-dose combination along with insulin in the management of type 2 diabetes mellitus: Indian experience. Cureus. 2021;13(1):
- Tripathi S, Tiwaskar M, Kota S, Parthan G, Dasgupta A, Mohanasundaram S, et al. Need of single pill fixed-dose combination with glimepiride in management of diabetes mellitus. J Assoc Physicians India. 2019;S1:30-3.
- Kim HS, Kim DM, Cha BS, Park TS, Kim KA, Kim DL, et al. Efficacy of glimepiride/metformin fixed-dose combination vs metformin uptitration in type 2 diabetic patients inadequately controlled on low-dose metformin monotherapy: a randomized, open label, parallel group, multicenter study in Korea. J Diabetes Investig. 2014;5(6):701-8.
- González-Ortiz M, Guerrero-Romero JF, Violante-Ortiz R, Wacher-Rodarte N, Martínez-Abundis E, Aguilar-Salinas C, et al. Efficacy of glimepiride/metformin combination versus glibenclamide/metformin in patients with uncontrolled type 2 diabetes mellitus. J Diabetes Complications. 2009;23(6):376-9.
- Sahay RK, Mittal V, Gopal GR, Kota S, Goyal G, Abhyankar M, et al. Glimepiride and metformin combinations in diabetes comorbidities and complications: real-world evidence. Cureus. 2020;12(9):e10700.
- Unnikrishnan AG, Pandit K, George J, Venkataraman S, Abhyankar MV. Clinical utilization pattern of multiple strengths of glimepiride and metformin fixed dose combinations in Indian type 2 diabetes patients. J Assoc Physicians India. 2020;68(7):57-61.
- Basnet A, Mahato B, Shrestha BL, et al. Efficacy and safety of low dose glimepiride-metformin fixed dose combination [0.5 mg glimepiride + 500 mg metformin sustained release (SR)] in patients with type 2 diabetes mellitus (T2DM) in Nepal. Indian Medical Gazette. 2019;
CLIII(6):94-8.
- Park CY, Kang JG, Chon S, Noh J, Oh SJ, Lee CB, et al. Comparison between the therapeutic effect of metformin, glimepiride and their combination as an add-on treatment to insulin glargine in uncontrolled patients with type 2 diabetes. PLoS One. 2014;9(3):e87799.