Abstract
Background: The prevalence of diabetes has been rising among the younger population and is a cause for concern. The present case-based questionnaire survey evaluated the treatment pattern and clinical experience of healthcare professionals (HCPs) in prescribing glimepiride/metformin fixed-dose combination (FDC) to young diabetes patients (up to 40 years of age) in the Indian setting. Material and methods: A retrospective, multicenter, observational, questionnaire-based survey was conducted in Indian healthcare centers using medical records of patients having type 2 diabetes mellitus (T2DM), who were prescribed different strengths of glimepiride/metformin FDCs. Data was collected from the patients’ medical records and were analyzed using statistical tests. Results: A total of 2,715 patients aged between 18 and 40 years were included in the study. Mean diabetes duration among the young patients was 2.76 ± 1.97 years. Among the young T2DM patients, 83.2% patients received glimepiride/metformin FDC as first-line therapy, and 16.8% received it as second-line therapy. Hypoglycemia at 6 months was noted in only 2.47% of the young patients. Mean glycated hemoglobin (HbA1c) before and after treatment was 8.7% ± 3.4% and 7.3% ± 3.9%, respectively. Mean fasting plasma glucose (FPG) was 171.8 ± 80.1 mg/dL in patients prior to treatment initiation and came down to 122.8 ± 41.8 mg/dL after treatment with glimepiride/metformin FDC. Mean postprandial plasma glucose (PPG) prior to combination therapy use was 248.7 ± 64.0 mg/dL and dropped to 177.2 ± 39.9 mg/dL after treatment. Good to excellent efficacy and tolerability were reported for 86% and 86.6% patients, respectively. Conclusion: This case-based questionnaire survey demonstrates the usage pattern of various strengths of glimepiride/metformin FDCs and the HCPs’ practice approach regarding the use of this combination in young T2DM patients in the Indian setting. The combination is commonly prescribed to young diabetes patients in India and is associated with beneficial effects on glycemic parameters.
Keywords: Type 2 diabetes, young adults, glimepiride/metformin combination, combination therapy
The prevalence of diabetes has been rising among the younger population and is a cause for concern as development of diabetes at a young age is linked with longer exposure to high blood glucose levels and an increased risk for complications during the lifetime. It also impacts the patient’s work and quality of life during the productive years.1 Indian data have shown a prevalence of type 2 diabetes mellitus (T2DM) between 1.1% and 4.7% among patients aged 30 years or below. There has been an increasing trend particularly over the past 10 years.2
Further, evidence has been strengthening that onset of T2DM at a young age is tied to a more aggressive disease phenotype.1 Insulin deficiency has been identified as the major factor accountable for T2DM in
young Indians, unlike their European counterparts in whom obesity and insulin resistance are the key drivers.3 Considering the fact that a rising number of young adults are being diagnosed with diabetes, the recommendations for screening have undergone revision. The United States Preventive Services Task Force (USPSTF) recommended lowering the age of screening in the United States from 40 to 35 years. However, it recommends screening at an earlier age in people belonging to groups with a higher prevalence, with special emphasis on screening Asian Americans at lower age as well as body mass index (BMI). It is thus suggested that screening should be initiated at age
25 years for nonpregnant adults in India and must focus overweight and obese individuals and people with a positive family history.2
Considering this, the treatment of young-onset T2DM must target a reduction of complications. The available data hints at the fact that tight glycemic control reduces the risk of microvascular complications.4 Metformin, one of the most extensively prescribed agents for T2DM management, is recommended for use in individuals aged above 10 years. Therapy starts at ages 10 to 16 years with 500 mg/day, and the dosage can be increased to 500 mg every 1 to 2 weeks, until a maximum dose of 2000 mg.4
Glimepiride is a sulfonylurea (SU) which has been reported to be tied to a low rate of hypoglycemia in adults. Besides affecting the pancreatic b-cell function, the agent also works by improving tissue sensitivity to insulin, with a favorable safety and efficacy profile.5 Interestingly, glimepiride has been shown to be as effective as metformin in reducing glycated hemoglobin (HbA1c) in young T2DM patients.5 A SU, glimepiride in particular, is the preferred drug to be used in combination with metformin in patients with diabetes.6,7 Adding a SU to metformin is preferred as the combination targets insulin resistance as well as insulin deficiency. Moreover, in resource-limited settings like India, SUs are cheaper than several other oral hypoglycemic drug classes, and effective as well.7
A study by Devarajan et al compared the safety and efficacy of glimepiride and sitagliptin in combination with metformin in T2DM patients and revealed that glimepiride/metformin combination led to significant reduction in glycemic parameters in comparison with sitagliptin/metformin combination.8
A case-based questionnaire survey conducted in Indian T2DM patients noted that different strengths of glimepiride/metformin fixed-dose combinations (FDCs) are safely prescribed in the young and the elderly population.9 A post-marketing surveillance study conducted in Nepal also showed beneficial effects of glimepiride/metformin FDC in young adults with T2DM, with improvements in glycemic parameters after 3 months of treatment.10
Glimepiride/metformin combination can also be used along with insulin therapy. In a study, the commonly prescribed oral antidiabetic drug combinations to be used as add-on with insulin glargine in patients with uncontrolled T2DM were assessed.
The result showed that the combination therapy of metformin and glimepiride with insulin significantly improved overall glycemic control, in comparison with other combinations.11 A real-world study conducted in India also noted good HbA1c reduction with glimepiride/metformin combination with insulin, and good to excellent efficacy and tolerability in patients across different age groups, including the young adults.12
Their proven efficacy, safety profile, pleiotropic benefits and low-cost, make SUs the preferred choice for treatment of diabetes in South Asians, and among SUs, modern agents like glimepiride are the preferred agents.13
A combination of glimepiride and metformin is commonly used in clinical practice for the management of diabetes in the Indian T2DM patients.14 There is a need for physician opinion on glimepiride/metformin combination amongst young T2DM patients in the Indian setting. A case-based questionnaire survey was, therefore, designed to evaluate the demography, treatment pattern including duration and various dosages of glimepiride/metformin FDCs in the management of T2DM in young patients.
MATERIAL AND METHODS
Study Design
This was a retrospective, multicenter, observational, questionnaire-based survey. It was conducted with 372 healthcare professionals (HCPs) across different centers in India between July 2020 and May 2021. The study protocol was designed in accordance with the principles of the Declaration of Helsinki.
Study Population
Patients of both sexes, aged between 18 and 40 years, diagnosed with T2DM who received a glimepiride/metformin FDC in any strength were recruited in the study.
Data Collection
A case report format was developed to determine the pattern of use of different strengths of glimepiride and metformin combination with or without other oral hypoglycemic agents (OHAs) in young diabetes patients. The questionnaire was sent to 372 HCPs across India through an online portal. Link to the portal was shared through e-mail. Questions regarding demographic characteristics, such as age, sex, BMI, medical history, education, occupation, area of stay and economic class; duration of diabetes; comorbidities; prevention program initiated; biochemical measures, such as fasting plasma glucose (FPG), postprandial plasma glucose (PPG) and HbA1c levels; antidiabetic drugs used; antidiabetic drug up-titrations and down-titrations; weight change; hypoglycemic episodes and other adverse events during treatment, were included in the questionnaire. The HCPs filled in the information on the online portal. A descriptive analysis was performed with the data provided on the portal.
Statistical Analysis
Descriptive statistical analyses, including mean and standard deviation (SD) for continuous variables and count and percentage for categorical variables, was performed. Chi-square test/Fisher’s exact test was used to compare two categorical variables. All the reported p-values were two-sided and p-values <0.05 were considered to indicate statistical significance. All data entries and statistical analyses were performed by using SPSS® Version 23.0 software.
RESULTS
A total of 2,715 T2DM patients aged ≤40 years receiving different strengths of glimepiride/metformin FDC were included in this retrospective observational questionnaire-based analysis.
The mean (± SD) age of patients was 34.7 ± 5.5 years. Mean duration of diabetes was 2.76 ± 1.97 years. A majority of patients had diabetes duration of 0 to 5 years. Mean BMI of the study participants was 28.12 ± 4.47 kg/m2. Table 1 summarizes the association of age with duration of diabetes.
According to the level of education, 41.3% patients were graduate, 27.3% had studied till or below 10th standard, 21.2% had studied till 12th standard and 10.2% had a postgraduate degree. Around 9.9% of the patients were unemployed, 19.9% worked in private service and 7.2% worked in government service, among other occupations. Based on the area of stay, 16.8% of the patients were from rural areas, 21.2% were from semi-urban areas, 27.6% were from urban areas and 34.5% were from metropolitans. Majority of the patients belonged to the upper-middle, higher-middle and the rich/elite economic class (70.7%).
Table 2 summarizes the patient demographics in terms of education, occupation, area of stay and economic class.
In this study, we could see that most patients belonged to the urban and metropolitan areas and the upper-middle, higher-middle and rich economic class.
Diabetes knowledge was average in 14.4% patients, fair in 2.6%, good in 45.5%, very good in 26.6% and excellent in 9.8% (result was not available for 1.1% of the patients).
A total of 2,258 patients (83.2%) received glimepiride/metformin FDC as first-line therapy, and 457 patients (16.8%) received it as second-line therapy. The most commonly prescribed glimepiride/metformin regimen was glimepiride 0.5 mg + metformin 500 mg (33.3%). Table 3 summarizes the dosage regimens used in the study participants.
Overall, 1097 (40.4%) patients also received another OHA with glimepiride/metformin FDC. About 153 patients (5.6%) received insulin along with glimepiride/metformin therapy, and 576 patients (21.2%) received concomitant medications, such as antihypertensives, antiplatelets, statins, calcium, methylcobalamin, etc. In the young T2DM patients, up-titration of glimepiride/metformin FDC was done in 32.5% and down-titration was done in 8.7%. Hypoglycemia at 6 months was evident in 2.5% patients. There were no other major adverse events.
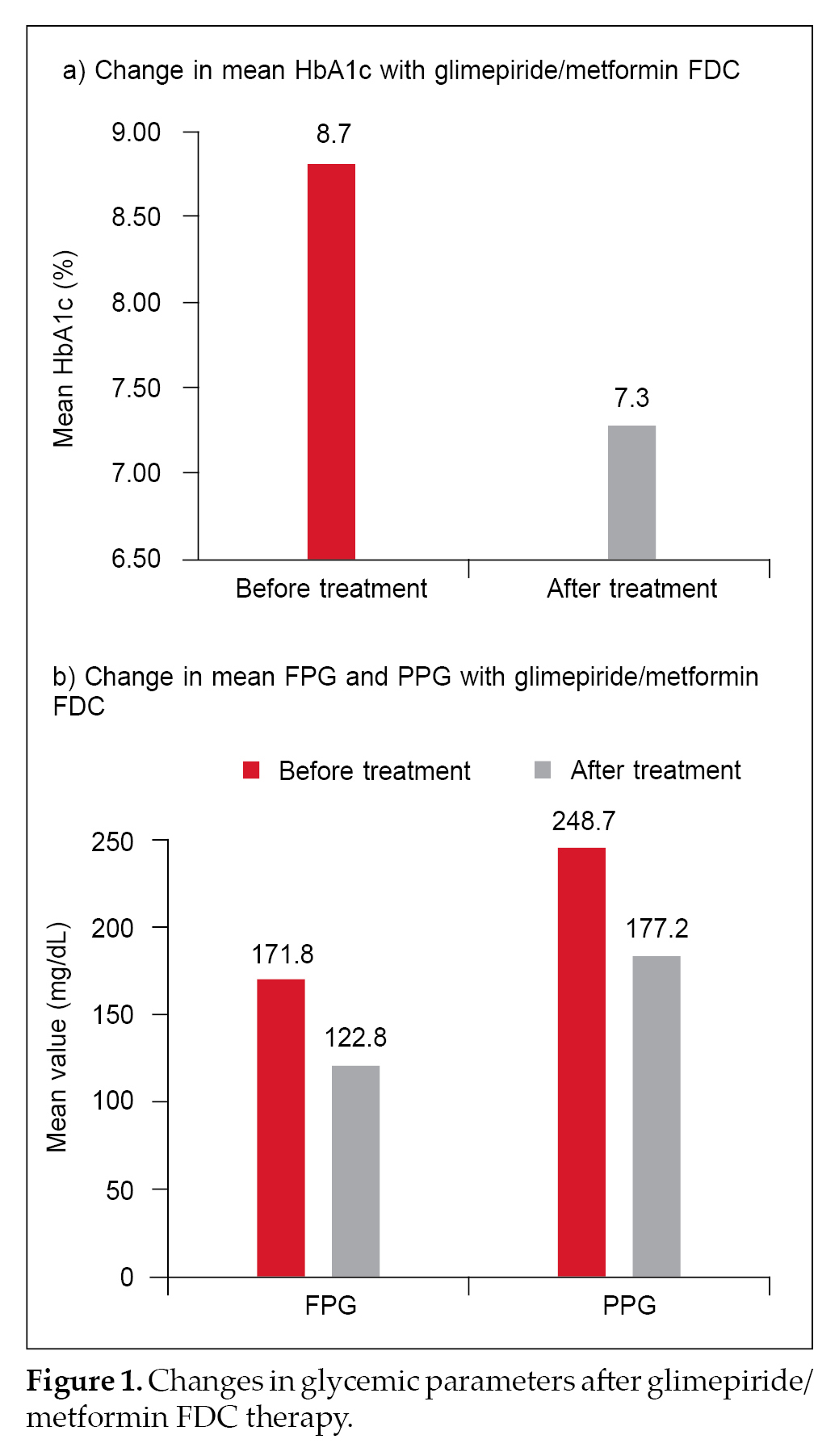
Mean HbA1c before treatment initiation was 8.7% ± 3.4% and decreased to 7.3% ± 3.9% after treatment with glimepiride/metformin FDC therapy. Mean FPG before initiating glimepiride/metformin FDC was 171.8 ± 80.1 mg/dL and came down to 122.8 ± 41.8 mg/dL after treatment. Mean PPG prior to combination therapy use was 248.7 ± 64.0 mg/dL and dropped to 177.2 ± 39.9 mg/dL
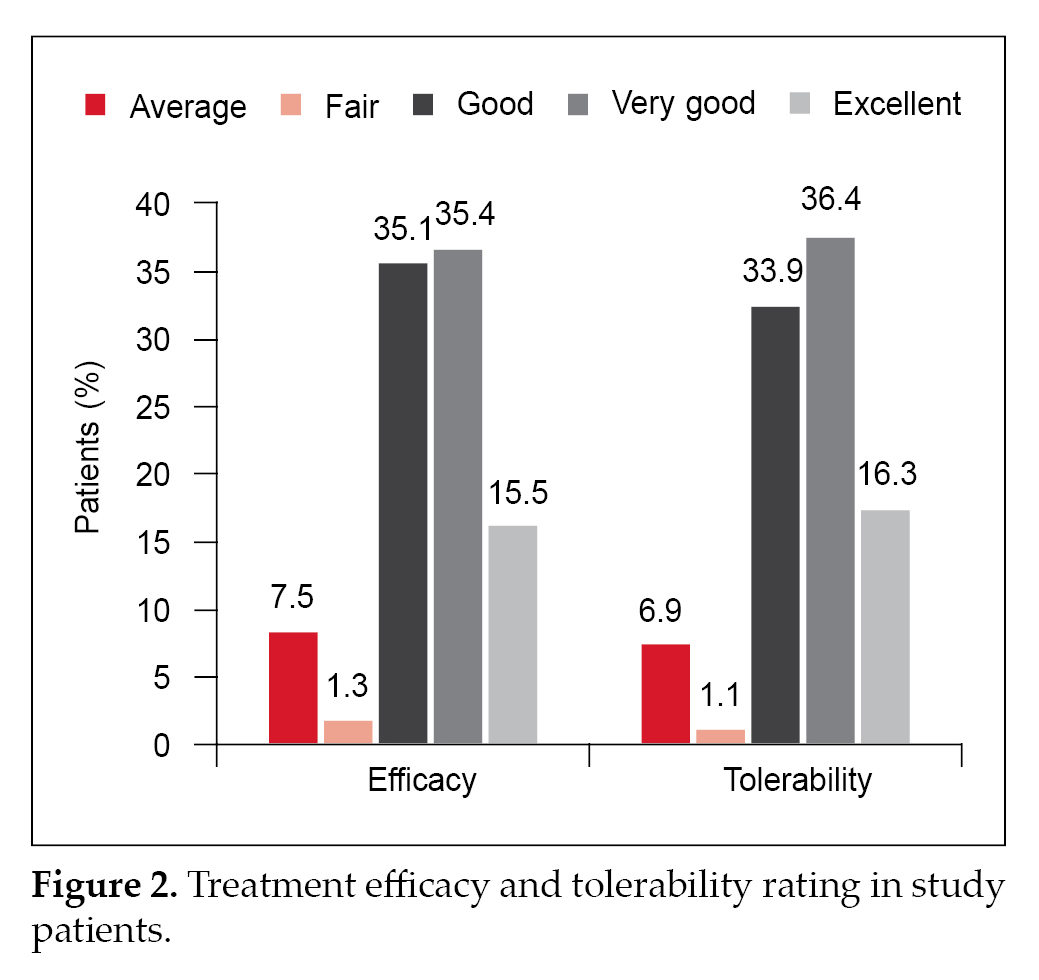
after treatment. Changes in the glycemic parameters are depicted in Figure 1 a and b. Physician evaluation of efficacy and tolerability were reported as good to excellent in 86% and 86.6% patients, respectively (Fig. 2).
|
Table 1. Patients in Different Age Groups Based on Diabetes Duration
|
|
Age group (years)
|
Diabetes duration (years)
|
|
0-5
|
6-10
|
11-15
|
>15
|
|
18-20
|
100 (3.94)
|
5 (3.01)
|
1 (12.5)
|
0 (0)
|
|
21-25
|
120 (4.73)
|
3 (1.81)
|
0 (0)
|
0 (0)
|
|
26-30
|
302 (11.9)
|
16 (9.64)
|
2 (25)
|
0 (0)
|
|
31-35
|
582 (22.94)
|
30 (18.07)
|
1 (12.5)
|
0 (0)
|
|
36-40
|
1433 (56.48)
|
112 67.47)
|
4 (50)
|
4 (100)
|
The values are mentioned as no. of patients (%).
|
Table 2. Demographics of the Patients Included in the Study
|
|
Variable
|
Number of patients (%)
|
|
Education
|
|
|
≤10th standard
|
741 (27.3)
|
|
12th standard
|
576 (21.2)
|
|
Graduate
|
1,122 (41.3)
|
|
Postgraduate
|
276 (10.2)
|
|
Occupation
|
|
|
Government service
|
196 (7.2)
|
|
Manual laborer
|
140 (5.2)
|
|
Private service
|
539 (19.9)
|
|
Professional
|
292 (10.8)
|
|
Self-employed
|
393 (14.5)
|
|
Semi-skilled
|
849 (31.3)
|
|
Unemployed
|
270 (9.9)
|
|
Any other
|
36 (1.3)
|
|
Area of stay
|
|
|
Rural
|
456 (16.8)
|
|
Semi-Urban
|
575 (21.2)
|
|
Urban
|
748 (27.6)
|
|
Metropolitan
|
936 (34.5)
|
|
Economic class
|
|
|
Poor
|
101 (3.7)
|
|
Lower-middle
|
693 (25.5)
|
|
Upper-middle
|
933 (34.4)
|
|
Higher-middle
|
387 (14.3)
|
|
Rich/elite
|
601 (22.1)
|
|
Table 3. Different Glimepiride + Metformin Dosage Regimens Used in Young Participants
|
|
Glimepiride/Metformin FDC regimen
|
Number of patients (%)
|
|
Glimepiride 0.5 mg/Metformin 500 mg
|
905 (33.3)
|
|
Glimepiride 1 mg/Metformin 500 mg
|
685 (25.2)
|
|
Glimepiride 2 mg/Metformin 500 mg
|
638 (23.5)
|
|
Glimepiride 1 mg/Metformin 850 mg
|
83 (3.1)
|
|
Glimepiride 2 mg/Metformin 850 mg
|
67 (2.5)
|
|
Glimepiride 3 mg/Metformin 850 mg
|
53 (1.9)
|
|
Glimepiride 0.5 mg/Metformin 1000 mg
|
59 (2.2)
|
|
Glimepiride 1 mg/Metformin 1000 mg
|
93 (3.4)
|
|
Glimepiride 2 mg/Metformin 1000 mg
|
114 (4.2)
|
|
Glimepiride 3 mg/Metformin 1000 mg
|
10 (0.4)
|
|
Glimepiride 4 mg/Metformin 1000 mg
|
8 (0.3)
|
DISCUSSION
Diabetes usually affects individuals above the age of 50 years in high-income countries, while in middle-income countries, the prevalence appears to be higher in young individuals. The young population in India is at a high risk for diabetes.15 Therefore, early aggressive treatment is needed in this population.16
The present case-based questionnaire survey explored the usage of glimepiride/metformin FDC in young patients with T2DM. This study assessed the approach of HCPs across India regarding the use of this combination in young patients with T2DM. A total of 2,715 patients were aged between 18 and 40 years were included in the study.
Although the trends are changing, diabetes is still more prevalent in urban areas. In this study also, somewhat similar findings were noted as only 16.8% of the patients were from rural areas. A recent study conducted in India among young adults (aged <35 years) noted that based on the Indian diabetes risk score (IDRS), the urban young population has a higher risk of diabetes compared to its rural counterparts.15
About 70.8% of the patients in this study belonged to the upper-middle, higher-middle and the rich/elite economic class. An increased prevalence of diabetes mellitus was noted in the higher social class in the Chennai Urban Rural Epidemiology Study (CURES-116) also.17
Around 83.2% of the young patients in the study received glimepiride/metformin FDC as first-line therapy, while 16.8% received it as second-line therapy. The combination of glimepiride and metformin is extensively used for controlling blood glucose levels on account of the ability of this combination to offset insulin secretion disorder as well as insulin resistance.14 Considering the fact that Asians develop diabetes at a relatively younger age compared to their Western counterparts, and at a lower BMI too, it has been suggested that the pathophysiological differences between Asians and Caucasians should be taken into account and only metformin should not be considered as the primary drug. In fact, all possible medications should be considered based on patient characteristics.18
There is ample real-world evidence to show that glimepiride/metformin combination is widely prescribed in T2DM patients.14 Metformin, a biguanide, acts by suppressing the basal hepatic glucose uptake and enhancing insulin-mediated glucose uptake in peripheral muscles. It does not stimulate insulin secretion. Therefore, its use is not tied to episodes of hypoglycemia. Hence, it is widely used in children and adolescents with diabetes. Likewise, the modern SU glimepiride is also tied to a low hypoglycemia rate in adults. The drug has an impact on pancreatic b-cell function.5
Like adult T2DM patients, children and adolescents or the young patients also develop diabetes due to insulin resistance and pancreatic b-cell secretory failure. The proven efficacy of OHAs in adults and the similar mode of disease development in the younger patients and in adults points to the fact that these agents will show similar efficacy in the younger patient population.5
Glimepiride and metformin combinations can be effectively used for both early and long-standing diabetes.14 It is noteworthy that within the class of SUs, glimepiride appears to be a better agent, compared to other SUs, used in combination with metformin.
A study has reported glimepiride/metformin combination to be more effective than glibenclamide/metformin combination to attain glycemic control.19 Moreover, early combination therapy with glimepiride and metformin is associated with the benefit of legacy effect on account of early glycemic control while evading a negative glycemic memory linked with micro- and macrovascular complications.18
In addition, modern SUs, such as glimepiride, have a cardiovascular-neutral profile. The CAROLINA trial found glimepiride to be at par with the dipeptidyl peptidase-4 (DPP-4) inhibitor linagliptin in terms of a risk of a composite cardiovascular outcome in T2DM patients with a high cardiovascular risk.20 Meanwhile, metformin has protective effects on several organs, especially the insulin-targeted tissues, including liver, muscles and adipose tissues. It also protects T2DM patients against cardiovascular diseases.21 Therefore, a combination of glimepiride and metformin seems to be a suitable therapeutic approach for young T2DM patients.
There have been quite a few studies which have corroborated the extensive use of this potent combination in diabetes patients with favorable outcomes, both in elderly and in young patients. A recent case-based questionnaire survey conducted by Unnikrishnan et al
evaluated the clinical utilization pattern of different strengths of glimepiride/metformin FDCs in patients with T2DM. The investigators concluded that various strengths of glimepiride/metformin FDCs are effective in diabetes patients, regardless of their age, diabetes duration, BMI, complications and use of concomitant medications.9 A post-marketing surveillance study conducted in Nepal showed the beneficial effects of the combination, particularly in the young patients. Among young T2DM patients (<40 years of age) receiving a glimepiride/metformin FDC (0.5 mg glimepiride + 500 mg metformin) noted an average reduction of 25% in FPG and a reduction of 43% in PPG after 3 months of therapy.10
Dose up-titration was done in 32.5% of the patients and down-titration was done in 8.7% of them in this study. Combinations of OHAs have helped clinicians a lot on account of the ease of up- and down-titration associated with their use.14
Like the study conducted in young T2DM patients in Nepal which evaluated the effect of glimepiride 0.5 mg + metformin 500 mg and noted potential benefits of the regimen,10 in the present study also, the most commonly prescribed glimepiride/metformin regimen in the young patients was glimepiride 0.5 mg + metformin 500 mg. Around 33.3% of the patients received this regimen.
Additionally, similar to other studies conducted with glimepiride/metformin combination,10 the present study also noted the beneficial effects of this combination in terms of glycemic parameters. There was a reduction in the key glycemic parameters after glimepiride/metformin FDC therapy.
Hypoglycemia at 6 months was noted in only 2.5% of the patients. This is even lesser than that seen in the study by Unnikrishnan et al, where 5.8% patients on glimepiride/metformin FDC therapy had hypoglycemia.9 In a real-world study, which evaluated the use of glimepiride/metformin combination along with insulin in diabetes patients, hypoglycemic events were noted in 6.1% of the patients.12 A limitation of this study is its retrospective nature. The strengths of the study include the information gathered on key glycemic parameters like HbA1c, FPG and PPG in young patients, which can be of great help in further evaluating the effects of this combination in young T2DM patients. The findings of this retrospective study should be further validated in large-scale prospective observational studies in order to achieve a better understanding of the efficacy and safety of glimepiride and metformin combination in this patient population in the Indian scenario.
CONCLUSION
This case-based questionnaire survey of the usage of glimepiride/metformin FDC in the Indian setting shows that multiple strengths of glimepiride/metformin FDCs are prescribed in young patients with T2DM. There was a significant improvement in glycemic parameters and fewer hypoglycemia episodes with this combination these patients. It can be concluded that glimepiride/metformin FDC is extensively prescribed to diabetes patients, even in the younger population, and is associated with beneficial effects on glycemic parameters. It would be appropriate to state that glimepiride/metformin FDC is suitable for the young as well as the elderly.
Authorship
All authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements
The authors would like to acknowledge Mr A Thamburaj and Ms Shashikala Borhade from USV Pvt. Ltd. For their assistance in the conduct of the project.
Contributors
K Ram Vijay Kumar, Meghanath Yenni, Kelli Chinna Babu, M Malleswara Rao, SDM Sekhar, Yalamanchi Sadasiva Rao,
ANS Varaprasad, K Vamsi Krishna, Hanumakumar S,
M Ramesh Kumar, A Jaswanth, J Amarendra, V Venkata Rama Kumar Vengal P Reddy, Anil Kumar Reddy I, Madhav Pavuluri, EA Shahul Hameed, N Karthikeyan, Shakeel Ahamed, J Senthil Kumar, S Palanivelrajan, P Kamalakannan, Vidhya Thambirajah, VN Selvam, A Lawrence Victor Joe, R Umarani, M Suriyakumar, Ashok M Kumar, Yerrathota Balaji, Nitchenametla Srinivas, Sepuri Krishna Mohan, Mallikarjuna Reddy N, Gaddale Rama Chandra Rao, Bandari Srinivasulu, Kumbha Srinivasa Rao, Harinath Reddy M,
N Nandagopal, Pasham Indrasen Reddy, Balasubrahmanyam K, N Sathyanarayana, U Rajanikanth, Mohd Shaeq Mirza, Rajesh Regonda, D Mathru, Perumalla Varunkumar,
G Naveen Kumar, D Jamal, P Sasi Kanth Reddy, Smitha Nalla, Manoj Kumar Dash, Chandrakanta Jha Choudhary, Pramod Pola, Harini Reddy, Siddanna D Kamat, Shridhar Balavanth Kulkarni, Niteen Chandrakant Shettye, Kushal D Godkhindi, Vinay M Dipali, Gajendra Mahishale, Ganesh HK, D Ganekal Prashanth, Vinay P Swamy, N Kotresh, BK Sundar, Ajit R Kulkarni, Basavaraj G Mangshetty, Manjunath G Anakal, Shashank Kulkarni, Arunkumar KB, Pruthvi
BC, Halli Karibasappa, Sadashivappa Chandrashekar,
Sabeer TK, Shakkeel Vea, Shankara BV, Sandeep Sudhakar, K Arumugam, Deyin Antony, Sanil Chandrasekharan, MP Senthilkumar, M Prasath, Manoj T Koshy, A Panneerselvam, PD Aravindan, Vijith Kumar K, N Sugumar, D Venkatesh, K Eswaran, Chandramohan P, Eswaran Thangavelu, R Arul Prakash, R Balamurugan, T Arunkumar, AS Arul, Vinoth K, Waseem Ahmed N, V Mahadevan, J Murali, C Jagadeesh, Karthik S, Rajkumar M, Nallaperumal S, Ravikumar V, Ambanna Gowda, MN Ashok, Ashok SN, Mala Dharmalingam, V Shankar, Sanjay S Rao, SG Harish, Rajeev H, Anantha Padmanavan, Aravinda J, Chikkalingaiah, NS Shiva Kumar, Ranjith A Shetty, Ravishankar SN, Supreeth
SK, Durga Prasad Bhimala, D Narayan Reddy, Bandaru Giri Prasad, D Sreevani, Metta Madhu, Suresh Munirathnam, Kavya J, Ch Lakshmi Jagadeesh, Rachamallu Ravichandra Reddy, Madhusudan Reddy K, G Satya Sreenivasa Rao, P Hari Kumar Reddy, M Leelavathi, K Chandra Obul Reddy, Potham Satish Kumar,A Praveen Kumar Reddy, BVS Ravi Shankar, P Mithun Chakravarthy, C Pradeep, L Sudheer Reddy, Gopala Venkata Giridhar, C Mallikarjun, B Raghavendra Rao, Ponnaian John Christopher, Navoday Gilla, Thummalcheral Dhanraju, Jaisimha Reddy, K Chandramouli Reddy, G Kiran, Madineni Ranganatham, Ponnapati Devanand, Vallamkonda Deepak Kumar, G Kalayn Charavarthy, Ambarkar Venkatesh, M Sreekanth Reddy, Pothamsetty Ravikaladhar Reddy, Veera Reddy P, Ravi Kumar, Karra Hanumantha Reddy,
SG Moazam, P Amareshwer, Pantala Chakradhar, Amaravai Sridhar Reddy, Valluri Srinivas Reddy, Mohammad D Shafee, J Nagaraju, G Sampavan Kumar, G Sainath Reddy, Mohammed Abubaker, Sanjoy Paul, Naveed Md Syed, B Hari Kishan, Dilip Gude, Prabhurami Reddy, K Jayarami Reddy, Eranki Prabhakar Sastry, Rajesh K, Surya Pavan Reddy, Madhav Balkrishna Prabhu, Satish Patil, Shivanand Boodihal, Rajesh Seth, Rajeev Joshi, Dhareppa Tammanna Kokatnur, Ashok Pandappa Yenkanchi, Nitin B Agarwal, Kalinga BE, Lakshmi Narayan, Parakal Haridas Upadhya,
BA Hanumanthu, Amruth Nanaiah, Y S Ravi Kumar, Sanjeev Rao Girimaji, Ashok HG, GK Mahesh, Mallikarjun H, EM Surendra, Manoj P Joseph, Joe George, Raju A Gopal, A Jayabal, R Kumaresan, KV Asaithambi, TS Ramaswamy, MG Uvaraj, VJ Kunjuvareed Rajasekar, R Suresh Prabu, Renuka Sriram, Sudha Sathian, V Dineshkumar, A Asraf Ali, Suguna Priya, K Arun Karthik, Nachimuthu Kumar, Shankar Dhandapani, Pothiraju Naicker Subbiah, DR Karunanidhi, TR Sivagnanam, M Bhuvana Priya, PS Mansur, KM Jeyabalaji, Ananth S, R Latha, Niroop PK, C Arshad Akeel, CR Anand Moses, G Bhaskara Naidu, Jaikumar G, Arun R, K Muralidaran, Subramani I, GM Prasad, Lalitha R, Manjanna M, R Shashi Kumar, Shivaprasad KS, Surekha B Shetty, KV Srinivasa, KN Manohar, Noor Khan, Dr Arpandev Bhattacharyya, Niran Uthaiah, NS Ramesh, Karunesh Kumar HS, S Naganarasimhaiah, Nagaraj S, Srinivasa Yogan,
BS Sudhir, Guruprasad Udupi, P Nitish, Raghurama NK, Keerthy Shetty, BV S Reddy, AC Shyam, Chandrashekar N, Jayalakshmi C, Sai Pradeep, V Prasanna, MN Raju, KP Sridhar Vaidya, Giridhar Patil, Naveen Angadi, AN Ramesh, Mabben EVS, Subhash Chandra Jayaram Balleke, KP Balraj, Rahul PG, Umesh D, PS Badri, TS Karthik Reddy, P Sateeshkumar Raju, Ch Sreeharsha Varma, A Rajeev, K Venugopala Reddy, Siripurapu Kiran Kumar, Purna GA Sreeramaneni, Dosapati Ramesh, M Kavitha, S Vasanth, M Manju Bhargavi, Goutham Meher, V Ravikumar, Kanumuri Srinivas Rao, Shaik Ahmed, M Shivakumar, Haritha, S Suneetha, Pradeep N Shantagiri, Rajendra M Arya, Praveen Kumar, Nishad, G Manoj, Robin George M, SN Ganesha Moorthy, Sengottuvel S,
A Balachandran, T Dhanalakshmi, K Srinivas, R Srinivasan, Ravi GR, M Shanmuganantham, Paramesh S, Naseem K Sait, L Sreenivasa Murthy, G Ganesh, Prasanna Kumar K, N Srikanth, MR Vidhya, A Kamlesh, B Karthik Rao, Jagdish Singh, Somappa, Jayesh Dhirajlal Shah, K Nagesh, M Nagesh Prabhu, Vimal MV, D Jeyapal, Aditya Chowty, Tirthankar, Dr R Manjunath, Sanjay Kulkarni, RN Bhat, Javvaji Venkateswarlu, KV Chandra Mouli Reddy, N Bhavani, Jayprakash Appajigol, KL Udapudi, Sunil N Gayad, Praveen Kusubi, A Shanmugam, SR Panchamukhi, Sudhir Jambagi, Laxman Jambagi, S Vijaya Bhaskara Reddy, N Rajendra Prasad, GV Siva Reddy, Vikrant B Ghatnatti, Rajendran V, Anilkumar A Kustagi, M Murali Krishna, Nekkanti Narendra Prasad, B Suresh Babu, Vijay S Desai, Shivaprasad C, Vinu Abraham, V Sreekanth Reddy, AM Rao, Sai Kumar, SM Gooranavar, Babu Rajendra Nayak, U Rammohan Rau, Dipu KP, Munibalakrishna SB Murali Krishnan, Patakamuri Padmalatha, Jaganmani Sreekanth, Sridhar CN, Raghavendra Prakash, M Natarajan, Vijayakumar P, Sreekumar Thekkoot, Jaidev Sudagani, Sandeep K Reddy, Sunil NK, Raj Mohan L, V Rajendran, Sajith VS, VT Thomas, R Shobana, Joshy Joseph, Thekke Mavila Muralidharan, Krishnanunni Polakkulath,
L L Dhivya
Funding
The project has been funded by USV Pvt. Ltd.
Conflict of interest
There are no conflicts of interest to declare.
REFERENCES
- Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6(1):69-80.
- Misra A, Ramachandran A, Saboo B, Kesavadev J, Sosale A,
Joshi S, et al. Screening for diabetes in India should be initiated at 25 years age. Diabetes Metab Syndr. 2021;15(6):102321.
- Prasad RB, Asplund O, Shukla SR, Wagh R, Kunte P,
Bhat D, et al. Subgroups of patients with young-onset type 2 diabetes in India reveal insulin deficiency as a major driver. Diabetologia. 2022;65(1):65-78.
- Temneanu OR, Trandafir LM, Purcarea MR. Type 2 diabetes mellitus in children and adolescents: a relatively new clinical problem within pediatric practice. J Med Life. 2016;9(3):235-9.
- Gottschalk M, Danne T, Vlajnic A, Vlajnic A, Cara JF. Glimepiride versus metformin as monotherapy in paediatric patients with type 2 diabetes: a randomized, single-blind comparative study. Diabetes Care. 2007;30(4):790-4.
- Acharya KG, Shah KN, Solanki ND, Rana DA. Evaluation of antidiabetic prescriptions, cost and adherence to treatment guidelines: aprospective, cross-sectional study at a tertiary care teaching hospital. J Basic Clin Pharm. 2013;4(4):82-7.
- Lim PC, Chong CP. What’s next after metformin? Focus on sulphonylurea: add-on or combination therapy. Pharm Pract (Granada). 2015;13(3):606.
- Devarajan TV, Venkataraman S, Kandasamy N, Oomman A,
Boorugu HK, Karuppiah SKP, et al. Comparative evaluation of safety and efficacy of glimepiride and sitagliptin in combination with metformin in patients with type 2 diabetes mellitus: Indian multicentric randomized trial - START Study. Indian J Endocr Metab. 2017;21(5):745-50.
- Unnikrishnan AG, Pandit K, George J, Venkataraman S, Abhyankar MV. Clinical utilization pattern of multiple strengths of glimepiride and metformin fixed dose combinations in Indian type 2 diabetes patients. J Assoc Physicians India. 2020;68(7):57-61.
- Basnet A, Mahato B, Shrestha BL, et al. Efficacy and safety of low dose glimepiride-metformin fixed dose combination [0.5 mg glimepiride + 500 mg metformin sustained release (SR)] in patients with type 2 diabetes mellitus (T2DM) in Nepal. Indian Medical Gazette. 2019;CLIII(6):94-8.
- Park CY, Kang JG, Chon S, Noh J, Oh SJ, Lee CB, et al. Comparison between the therapeutic effect of metformin, glimepiride and their combination as an add-on treatment to insulin glargine in uncontrolled patients with type 2 diabetes. PLoS One. 2014;9(3):e87799.
- Prasanna Kumar KM, Seshadri K, Aravind SR, Deb P, Modi KD, Gopal RA, et al. Real-world observational study of glimepiride and metformin fixed-dose combination along with insulin in the management of type 2 diabetes mellitus: Indian experience. Cureus. 2021;13(1):e13020.
- Kalra S, Aamir AH, Raza A, Das AK, Azad Khan AK, Shrestha D, et al. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: a consensus statement. Indian J Endocrinol Metab. 2015;19(5):577-96.
- Sahay RK, Mittal V, Gopal CR, Kota S, Goyal G, Abhyankar M, et al. Glimepiride and metformin combinations in diabetes comorbidities and complications: real-world evidence. Cureus. 2020;12(9):e10700.
- Nagarathna R, Bali P, Anand A, Srivastava V, Patil S,
Sharma G, et al. Prevalence of diabetes and its determinants in the young adults Indian population-call for Yoga intervention. Front Endocrinol (Lausanne). 2020;11:507064.
- TODAY Study Group, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, et al. A clinical trial to maintain glycaemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247-56.
- Skar M, Villumsen AB, Christensen DL, Petersen JH, Deepa M, Anjana RM, et al. Increased risk of type 2 diabetes with ascending social class in urban South Indians is explained by obesity: the Chennai Urban Rural Epidemiology Study (CURES-116). Indian J Endocrinol Metab. 2013;17(6):1084-9.
- Kim HS, Kim DM, Cha BS, Park TS, Kim KA, Kim DL, et al. Efficacy of glimepiride/metformin fixed-dose combination vs metformin uptitration in type 2 diabetic patients inadequately controlled on low-dose metformin monotherapy: a randomized, open label, parallel group, multicenter study in Korea. J Diabetes Investig. 2014;5(6):701-8.
- González-Ortiz M, Guerrero-Romero JF, Violante-Ortiz R, Wacher-Rodarte N, Martínez-Abundis E, Aguilar-Salinas C, et al. Efficacy of glimepiride/metformin combination versus glibenclamide/metformin in patients with uncontrolled type 2 diabetes mellitus. J Diabetes Complications. 2009;23(6):376-9.
- Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, et al; CAROLINA Investigators. Effect of linagliptinvs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322(12):1155-66.
- Yang X, Xu Z, Zhang C, Cai Z, Zhang J. Metformin, beyond an insulin sensitizer, targeting heart and pancreatic β cells. Biochim Biophys Acta Mol Basis Dis. 2017;1863(8):1984-90.