Abstract
Background: To understand the approach of clinicians about the treatment pattern, dosage, efficacy and safety of the combination of low-dose glimepiride (0.5 mg) and metformin fixed-dose combination (FDC) in the management of type 2 diabetes mellitus (T2DM) continuum in Indian settings. Methods: This case-based questionnaire survey included health care professionals (n = 112) across India, who were prescribing glimepiride and metformin FDC. Data were collected from the medical records and analyzed. Results: The data of 1,403 patients with T2DM were included. The mean age was 49.1 years and 68.4% of patients were males. The median duration of T2DM was 36 months. A total of 86.7% of patients received glimepiride and metformin FDC as first-line therapy. The most commonly prescribed (71.5%) dosage of glimepiride and metformin was 0.5 mg/500 mg. The titration of the dose was performed in 231 patients, of which 82.7% required up-titration and 17.3% required down-titration. The mean glycated hemoglobin (HbA1c), fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) levels reduced significantly (mean change: 1.2%, 36.5 mg/dL and 50.2 mg/dL,
respectively) post-treatment. The hypoglycemic event and weight gain were reported in 7.7% and 9.5% of patients, respectively. Overall physician’s global evaluation of efficacy and tolerability was rated good to excellent in the majority of patients (>85%). Conclusion: Results demonstrate low-dose (0.5 mg) glimepiride and metformin FDC is effective in achieving glycemic control through lowering HbA1c, FPG and PPG levels with acceptable safety outcomes.
Keywords: Low-dose, fixed-dose combination, glycemic control, tolerability
Background
Type 2 diabetes mellitus (T2DM) is one of the most important public health concerns worldwide, affecting 537 million people in 2021. This number is expected to increase to 783 million by 2045.1 India harbors the second largest T2DM population – of 74.2 million cases in 2021 which is estimated to increase to 124.9 million cases by 2045.1
The primary goal of T2DM management is to achieve and maintain a good glycemic control to limit the long-term micro- and macrovascular complications.2 Therefore, early diagnosis and management are particularly important to lower blood-glucose levels aggressively and to reduce diabetes-related morbidity and mortality.
There are a number of antidiabetes agents available for managing T2DM.3 The choice of antidiabetes agents is based on efficacy along with drug safety. Metformin has been the most recommended monotherapy for the initial treatment of T2DM.4-6 However, many diabetic patients eventually require more than one drug, due to treatment failure with monotherapy
over time.7 Combined regimens are effective to minimize the dosage of antihyperglycemic agents and thereby their unwanted effects. Among various medications, combination therapy using modern sulfonylurea, (glimepiride) and metformin has shown to be effective in improving glycemic control.8,9
Early implementation of combination therapy using submaximal doses of glimepiride and metformin could improve glycemic control with marginal micro- and macrovascular complications.10 Glimepiride and metformin fixed-drug combination (FDC) has a complementary mechanism that promotes insulin secretion and improves insulin resistance,11 and its use has seen a rise worldwide.12 Glimepiride and metformin FDC is widely used in Indian clinical settings due to its efficacy and cost-effectiveness in improving glycemic control.8,13
Among glimepiride and metformin FDCs, the low-dose combination (0.5 mg + 500/1000 mg) is widely used and well-accepted.14,15 The glimepiride and metformin FDC (0.5 mg + 500 mg) is useful in patients with early-stage T2DM. The therapy was well-tolerated with no reports of hypoglycemia or weight gain.14 Evidence suggests that treatment with low-dose glimepiride (0.5 mg) and metformin can improve glycemic control in newly diagnosed patients and those with diabetes duration <5 years with a lower risk of hypoglycemic events and weight gain.8,15,16
However, data is lacking about the real-life practice of low-dose (0.5 mg) glimepiride and metformin FDC in treating Indian patients with T2DM. Therefore, the present study aimed to understand the clinician’s approach regarding the treatment pattern, the dosage used, and the efficacy and safety of low-dose glimepiride and metformin FDC in the management of the T2DM continuum in Indian settings.
Materials and Methods
Study design
This case-based questionnaire survey study included 112 health care professionals (HCPs) [general physician, endocrinologist, diabetologist, cardiologist and neurologist] who participated in online surveys, across India and prescribed glimepiride and metformin FDC for their patients with T2DM. This study was conducted between June 2020 and June 2021. Here, the HCPs provided information on these patients retrospectively.
Study population
The study population included patients of either sex aged >18 years who were diagnosed with T2DM and were taking or newly started glimepiride and metformin FDC. The data was collected from the medical records of all eligible patients from selected clinics.
Data collection
The questionnaire included questions regarding demographic characteristics (age, sex, education, occupation, weight, height, heart rate and blood pressure), risk factors, history of diabetes complications, biochemical measures (fasting plasma glucose [FPG] and postprandial plasma glucose [PPG], levels of glycated hemoglobin [HbA1c]), comorbidities, use of glimepiride and metformin FDC (first-line/second-line), the dosage of glimepiride and metformin FDC (0.5/500 mg or 0.5/1000 mg), frequency, initiation of a combination of glimepiride and metformin FDC at the levels of HbA1c, FPG and PPG, concomitant and antidiabetic medications (treatment pattern), dosage up-titration, and down-titration, reasons for up- and down-titration, lipid parameters, glycemic parameters changes, weight changes and hypoglycemic episodes during therapy. Patients having incomplete data files or with any condition that according to the discretion of the investigator indicated that the patient were not suitable for inclusion in the study, were excluded.
Outcomes
The primary objective was to study the demographics of patients receiving a dosage of glimepiride and metformin FDC and/or with other antidiabetic drugs in the management of T2DM. This study also assessed different doses of glimepiride and metformin FDC (0.5/500 mg or 0.5/1000 mg), HbA1c levels, up-titration and down-titration, weight changes, hypoglycemic episodes and comorbidities and complications during antidiabetic therapy.
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) software, version 23.0. Qualitative data were presented as numbers and percentages, while quantitative data were presented as mean (standard deviation [SD]) or median (range), depending on the normal or skewed distribution of data. A paired sample t-test was used for comparing the pre- and post-treatment HbA1c, FPG and PPG levels. A p < 0.05 was considered statistically significant.
Results
A total of 1,403 patients were included in the study, of which 68.4% of patients were males. The mean (SD) age was 49.1 (11.9) years and 54.8% of patients were aged between >41 to ≤60 years. The demographic characteristics are summarized in Table 1.
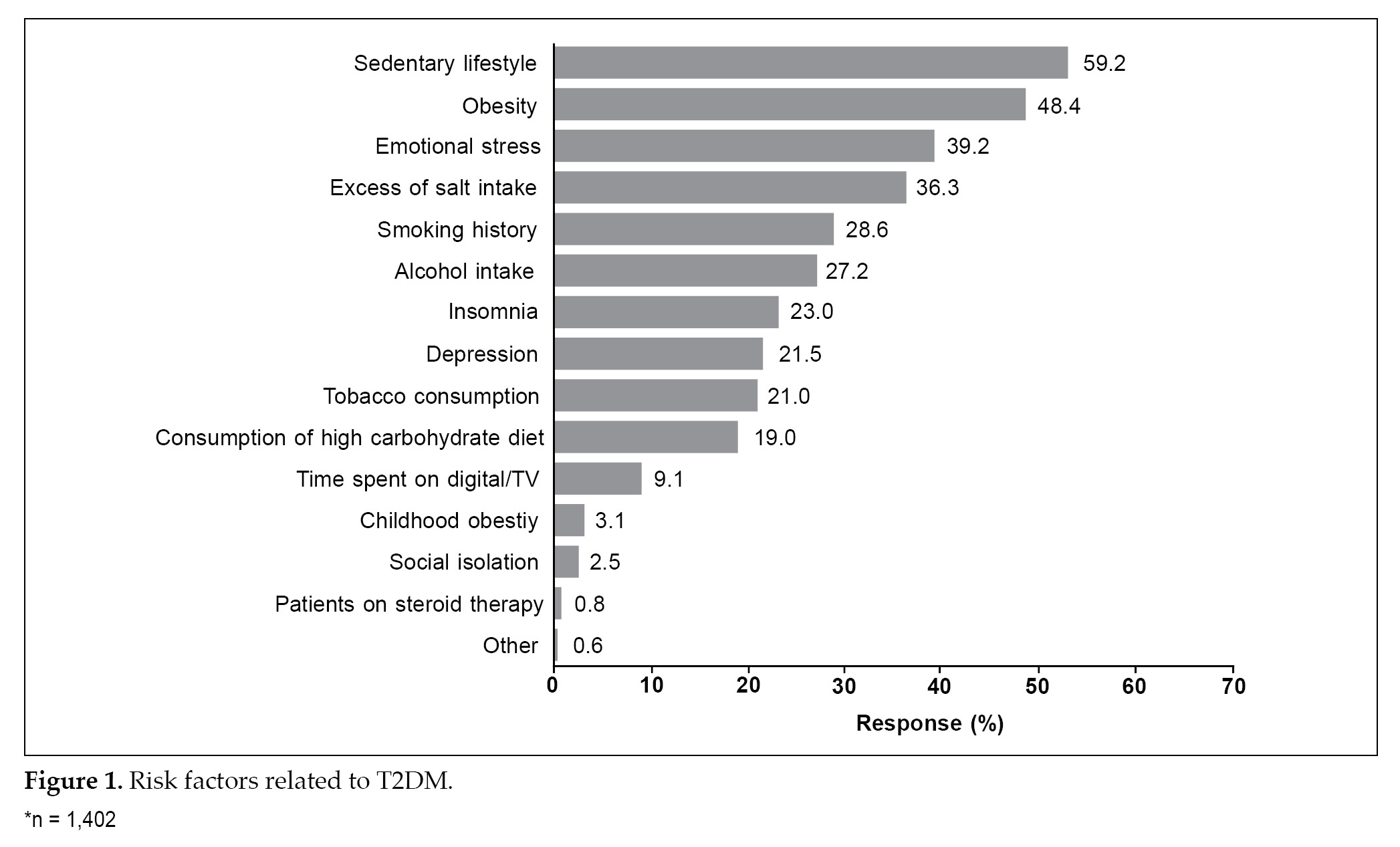
The median duration of T2DM was 36.0 months. Sedentary lifestyle (59.2%), obesity (48.4%) and emotional stress (39.2%) were the most common risk factors observed among these patients (Fig. 1). A family history was noted in 1,073 patients; of these, 671 patients were the first-degree relatives. Neuropathy was the most common complication observed in 21.7% of the patients.
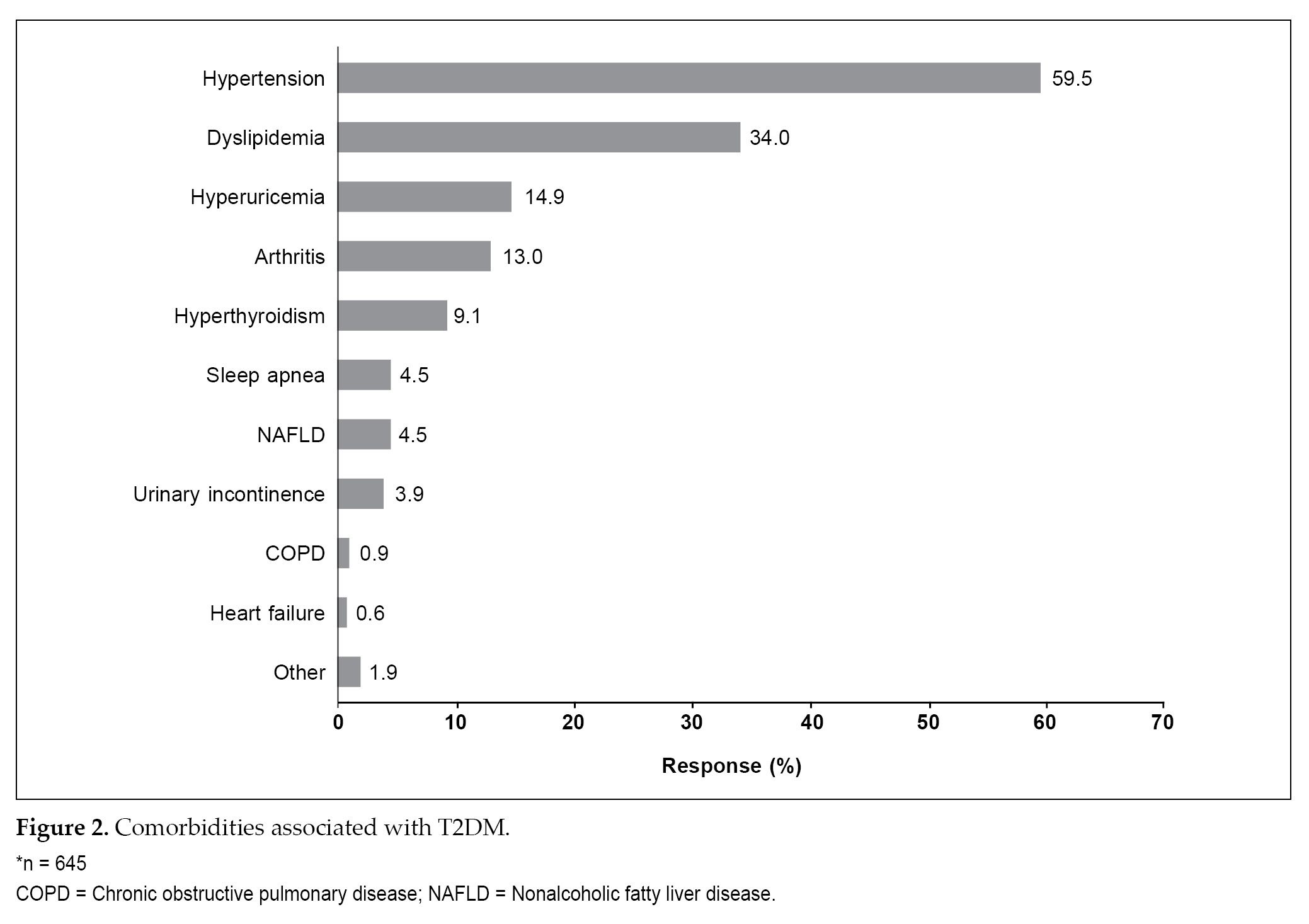
A total of 113 patients were current smokers while 59 were former smokers (Table 1). A total of 645 patients reported comorbid conditions, of these hypertension (59.5%) was the most common comorbidity, followed by dyslipidemia (34.0%) (Fig. 2).
A total of 86.7% of patients received glimepiride and metformin FDC as first-line therapy. The strength of glimepiride 0.5 mg and metformin 500 mg (71.5%) was the most commonly prescribed combination followed by glimepiride 0.5 mg and metformin 1000 mg
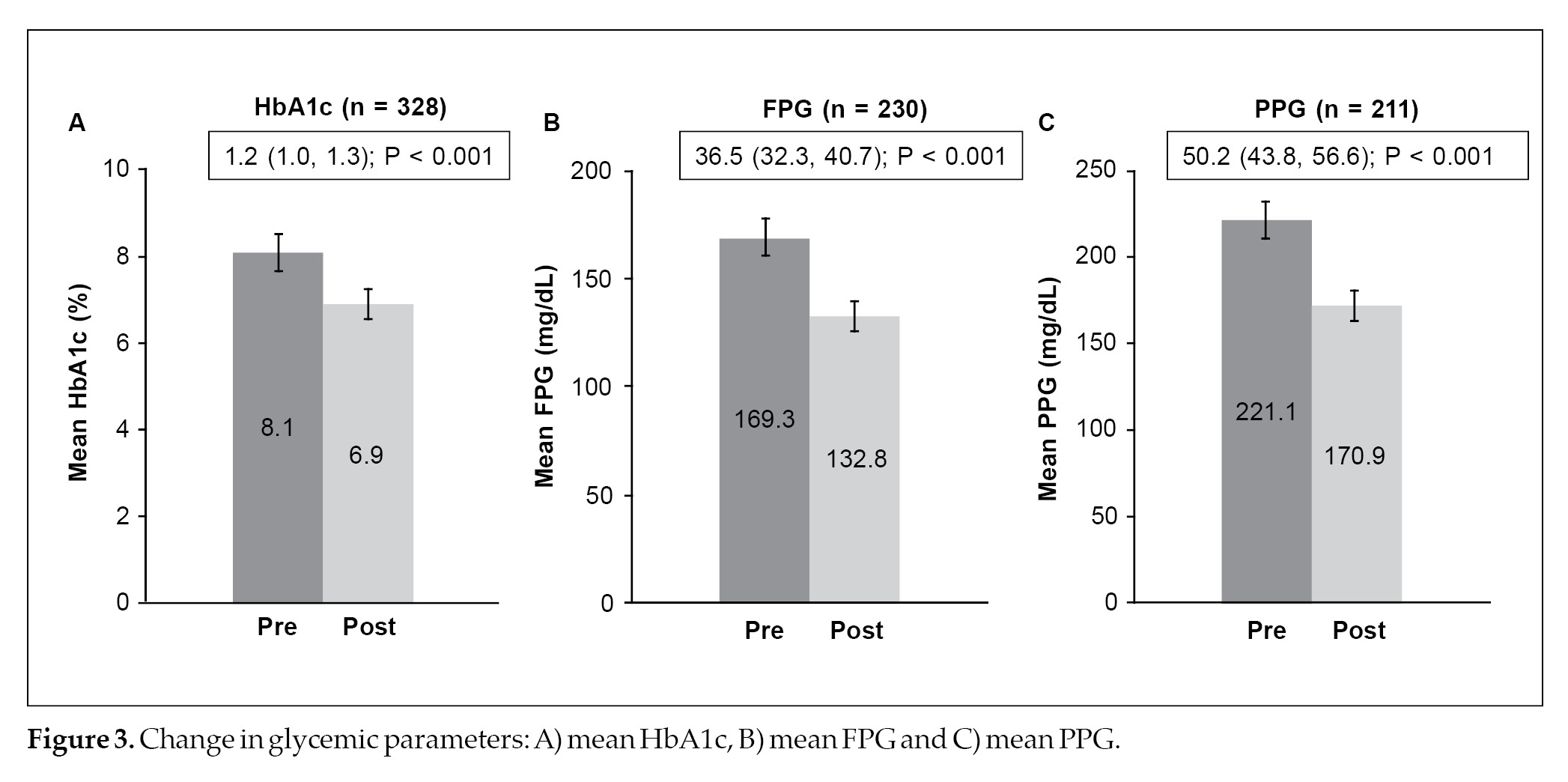
combination (28.5%). Once a day was the preferred dosing frequency in 733 (58.7%) patients with T2DM, while twice a day was prescribed in 515 (41.3%) patients with T2DM. The median duration of treatment of low-dose glimepiride and metformin FDC therapy was 23.4 months. Titration of the dose was performed in 231 patients and of these, 82.7% required dosage up-titration and the remaining required dosage down-titration (Table 2). The mean HbA1c levels significantly decreased post-treatment with glimepiride and metformin FDC – with a mean change of 1.2% (95% confidence interval [CI], 1.0-1.3; p < 0.001) (Fig. 3A). Similarly, the mean FPG (mean change: 36.5 mg/dL; 95% CI, 32.3-40.7; p < 0.001) and PPG (mean change: 50.2 mg/dL; 95% CI, 43.8-56.6;
p < 0.001) levels were significantly reduced post-treatment with glimepiride and metformin FDC therapy (Fig. 3B and 3C). Hypoglycemic events were reported in 7.7% of patients and weight gain was
|
Table 1. Demographic Characteristics
|
|
Parameters
|
Number of responses
(n = 1,403)*
|
|
Age (years), mean (SD)
|
49.1 (11.9)
|
|
Age groups (years)
≤40
>41-≤60
>61
|
405 (28.9)
769 (54.8)
229 (16.3)
|
|
Sex
Male
Female
|
960 (68.4)
443 (31.6)
|
|
Weight (kg)
|
74.4 (11.2)
|
|
Height (cm)
|
1.7 (0.1)
|
|
BMI (kg/m2)
|
27.1 (4.1)
|
|
Education (n = 1,287)
Up to 10th standard
Up to 12th standard
Graduate
Postgraduate
|
138 (10.7)
277 (21.5)
679 (52.8)
193 (15.0)
|
|
Occupation (n = 1,253)
Private service
Self-employed
Government servant
Unemployed
Professional
Semi-skilled
Manual laborer
House-wife
Retired
|
268 (21.4)
246 (19.6)
232 (18.5)
214 (17.1)
119 (9.5)
64 (5.1)
49 (3.9)
47 (3.8)
14 (1.1)
|
|
Area of stay (n = 1,256)
Semi-urban
Urban
Rural
Metropolitan
|
544 (43.3)
489 (38.9)
200 (15.9)
23 (1.8)
|
|
Economic class (n = 1,246)
Upper Middle
Lower Middle
Higher Middle
Poor
Rich/elite
|
529 (42.5)
444 (35.6)
180 (14.4)
54 (4.3)
39 (3.1)
|
|
Smoking status (n = 172)
Current
Former
|
113 (65.7)
59 (34.3)
|
|
Blood pressure (mmHg), mean (SD)
SBP
DBP
|
135.3 (17.4)
85.3 (8.8)
|
|
Duration of T2DM (months), median (IQR)
|
36.0 (18.0-72.0)
|
|
HbA1c (%), mean (SD)
|
7.7 (0.8)
|
|
Blood glucose (mg/dL), mean (SD)
FPG
PPG
|
157.8 (35.7)
236.4 (49.8)
|
|
Lipid parameter (mg/dL)
Total cholesterol (n = 189)
LDL (n = 162)
HDL (n = 155)
Triglycerides (n = 144)
|
198.0 (44.4)
132.3 (55.3)
45.1 (14.7)
166.4 (76.2)
|
|
FH (n = 1073)
FH of diabetes (First-degree relative)
FH of diabetes (Second-degree relative)
FH of obesity
|
671 (62.5)
320 (29.8)
227 (21.2)
|
|
Complications
Neuropathy
Retinopathy
CAD
Erectile dysfunction
PAD
TIA
Foot ulcer
Nephropathy
Other
|
304 (21.7)
192 (13.7)
155 (11.0)
46 (3.3)
42 (3.0)
28 (2.0)
24 (1.7)
2 (0.1)
10 (0.7)
|
Data shown as n (%), unless otherwise specified. *n = 1,403, unless otherwise specified.
BMI = Body mass index; CAD = Coronary artery disease; DBP = Diastolic blood pressure; FH = Family history; FPG = Fasting plasma glucose;
HbA1c = Glycated hemoglobin; HDL = High-density lipoprotein; IQR = Interquartile range; LDL = Low-density lipoprotein; PAD = Peripheral artery disease; PPG = Postprandial plasma glucose; SBP = Systolic blood pressure; SD = Standard deviation; T2DM = Type 2 diabetes mellitus; TIA = Transient ischemic attack.
observed in 9.5% of patients in the past 6 months (Table 2). A total of 34.7% (n = 487) patients received glimepiride and metformin FDC along with other antidiabetic medications. The majority of patients (n = 342) received dipeptidyl peptidase-4 (DPP-4) inhibitors along with glimepiride and metformin FDC. Insulin therapy was initiated in 54 (3.8%) patients along with glimepiride and metformin FDC (Table 3). The majority of the patients (54.1%) preferred digital platforms for accessing knowledge on diabetes (Table 4).
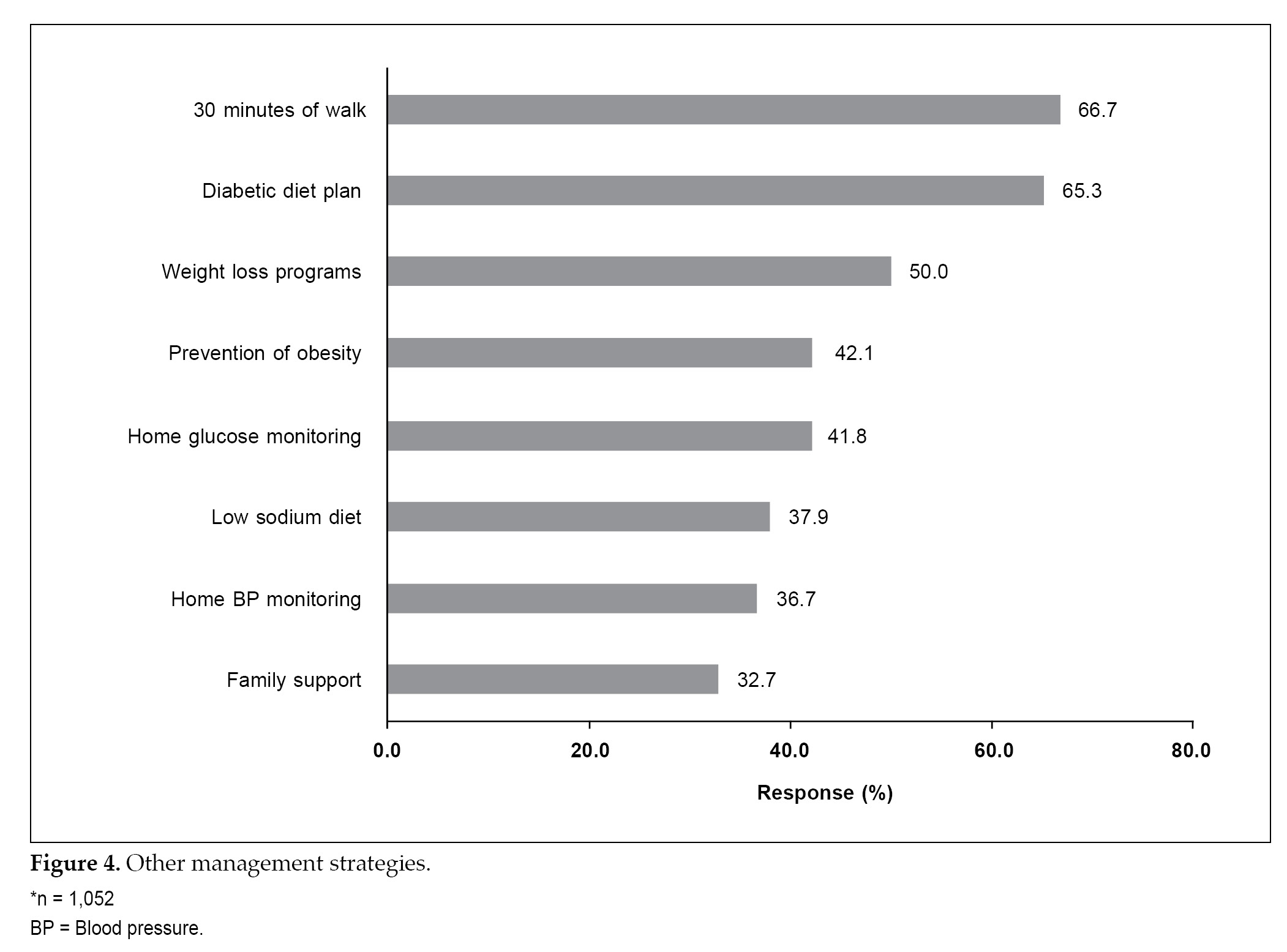
The most common managing strategies other than antidiabetes treatment included 30 minutes of walk (66.7%), diabetes diet plan (65.3%) and weight loss program (50.0%) (Fig. 4). Full compliance with
|
Table 2. Glimepiride and Metformin FDC Patterns and Post-treatment Parameters
|
|
Parameters
|
Number of responses (n = 1,403)*
|
|
Use of glimepiride and metformin FDC
First-line therapy
Second-line therapy
|
1,217 (86.7)
186 (13.3)
|
|
Treatment pattern of drug dosage
Glimepiride 0.5 mg + Metformin 500 mg
Glimepiride 0.5 mg + Metformin 1000 mg
|
1,003 (71.5)
400 (28.5)
|
|
Frequency of dose (n = 1,248)
OD
BID
|
733 (58.7)
515 (41.3)
|
|
Duration of glimepiride and metformin FDC therapy (months), median (IQR) [n = 1110]
|
23.4 (12.0-38.0)
|
|
Up-titration or down-titration of glimepiride and metformin FDC (n = 231)
Dosage up-titration
Dosage down-titration
|
191 (82.7)
40 (17.3)
|
|
Hypoglycemic event in the past 6 months
|
108 (7.7)
|
|
Weight gain
|
133 (9.5)
|
Data shown as n (%). *n = 1403, unless otherwise specified.
FDC = Fixed-dose combination; OD = Once daily; BID= Twice daily.
|
Table 3. Concomitant Antidiabetes Medications
|
|
Medications
|
Number of responses (n = 487)
|
|
Insulin therapy
|
54 (3.8)
|
|
DPP-4 inhibitors
|
342 (70.2)
|
|
SGLT2 inhibitors
|
100 (20.5)
|
|
Thiazolidinedione
|
78 (16.0)
|
|
AGIs
|
78 (16.0)
|
|
GLP-1 agonist
|
4 (0.8)
|
|
Data shown as n (%). *n = 1403, unless otherwise specified.
AGIs = Alpha-glucosidase inhibitors; DPP-4 = Dipeptidyl peptidase-4; GLP-1 = Glucagon-like peptide 1; SGLT2 = Sodium-glucose
co-transporter 2.
|
medication was observed in 42.4% of the patients while 27.6% and 18.3% of patients forgot medication once a month and once a week, respectively (Table 4).
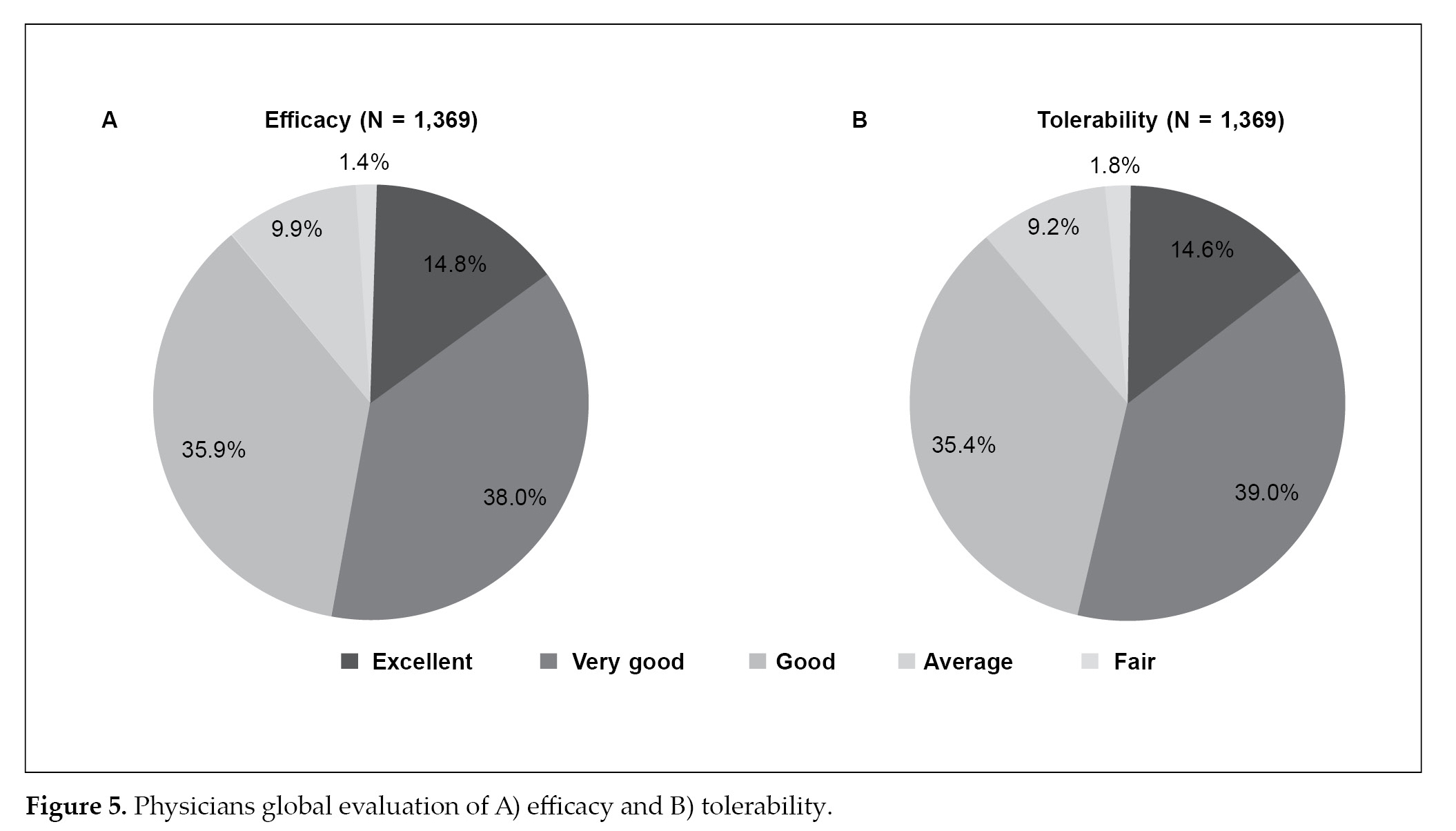
Physician global evaluation of efficacy (88.7%) and tolerability (89.0%) showed that a majority of patients were on a good to excellent scale (Fig. 5).
Discussion
The use of a single antidiabetic agent in patients with long duration of diabetes fails to achieve the glycemic targets. Hence, many patients require combination therapy in the long run, to achieve optimal control.7 Several guidelines recommended the use of early initiation of combination therapy in some patients to extend the time to treatment failure.17,18 Newer generation sulfonylurea (glimepiride) is the most frequently used add-on drug when the HbA1c target is not achieved by metformin monotherapy.10
According to a clinical trial conducted in Korean patients with T2DM – that compared glimepiride, metformin and rosiglitazone monotherapy, the reduction in HbA1c was comparable among the three drugs. Furthermore, it was shown that a half-maximal dose is sufficient to achieve a glucose-lowering effect.19 It seems advisable in patients with T2DM to use a combination of two drugs with different mechanisms of action than increasing the dose of monotherapy.19 And therefore, glimepiride, a third-generation sulfonylurea is widely prescribed in Asian countries and also in many other countries as a primary drug or as add-on, when patients do not reach their target HbA1c levels on metformin monotherapy.
The present study retrospectively evaluated the approach of clinicians regarding the treatment pattern, the dosage used, efficacy and safety of a combination of low-dose glimepiride (0.5 mg) and metformin (500/1000 mg) in the management of T2DM continuum in the Indian settings.
The key observations of the present study were: i) sedentary lifestyle, obesity and emotional stress are possible modifiable risk factors associated with T2DM; ii) family history was found to be the most common association; iii) neuropathy and hypertension were the most common diabetes complication and comorbid condition, respectively, in the majority of patients; iv) low-dose (0.5 mg) glimepiride and metformin FDC was
|
Table 4. Observations Related to Patient Care
|
|
Parameters
|
Number of responses
(N = 1,403)*
|
|
Diabetes knowledge sharing platform (n = 1,003)
Digital
Telephonic
Other
Consultation
Leaflet
Family support
Literature
|
543 (54.1)
436 (43.5)
24 (2.4)
11 (45.8)
10 (41.7)
2 (8.3)
1 (4.1)
|
|
Patients having knowledge about T2DM
Excellent
Very good
Good
Average
Fair
|
134 (9.6)
351 (25.0)
557 (39.7)
274 (19.5)
61 (4.3)
|
|
Exercise program (n = 1,273)
Regular
Moderate
|
732 (57.5)
541 (42.5)
|
|
Adherence to medications (n = 1,171)
Total compliance to medication
Forget medication once a month
Forget medication once a week
Partial compliance for medication
Forget medication once a year
|
496 (42.4)
323 (27.6)
214 (18.3)
130 (11.1)
114 (9.7)
|
|
Data shown as n (%). *n = 1403, unless otherwise specified.
|
prescribed as first-line therapy; v) the mean HbA1c, FPG and PPG levels decreased significantly after initiation of low-dose glimepiride and metformin FDC therapy demonstrating efficacy in terms of achieving glycemic target; vi) more than half of the patients achieved blood pressure and lipid levels within the normal range after treatment; vii) hypoglycemic events were reported in 7.7% of patients and weight gain was observed in 9.5% patients.
Considering the compliance and cost-effectiveness, the use of sulfonylurea and metformin FDC has recently increased.8 FDCs have been shown to improve patient compliance by reducing pill burden20 and is expected to provide better glycemic control with good durability and low risk of side effects. In the START study, the safety and efficacy of glimepiride (1 or 2 mg) and metformin (1000 mg) FDC once daily, compared with sitagliptin (50 mg) and metformin (500 mg) FDC twice daily, in patients with T2DM – who were either drug-naïve or uncontrolled on metformin. The mean HbA1c was significantly reduced from baseline in the glimepiride group compared to the sitagliptin group (0.42% vs. 0.30%; p = 0.001) at week-12. Moreover, FPG and PPG levels were significantly reduced in the glimepiride group.11
A multicentric, randomized study was conducted to compare the efficacy and safety of low-dose glimepiride and metformin (glimepiride 0.5 mg + metformin 500 mg) FDC in young adults (≤40 years) and those with early stage diabetes. The findings of this study showed a reduction in FPG (26%) and PPG levels (39%) post-glimepiride and metformin treatment with minimal hypoglycemic (8%) effects.15 Unnikrishnan et al reported various strengths of glimepiride and metformin FDC therapy for the management of T2DM in India irrespective of age, duration of diabetes, body mass index (BMI), diabetes complications and use of concomitant medications. Furthermore, authors alluded that low-dose glimepiride and metformin (glimepiride 0.5 mg + metformin 500 mg) FDC was not associated with hypoglycemic events.8 In the present study, a low-dose combination of 0.5 mg glimepiride with 500 or 1000 mg
metformin was effective in achieving glycemic target. This is in accordance with the study done by George J.14
Poor glycemic control and multiple cardiovascular risk factors can lead to the development of micro- and macrovascular complications in patients with T2DM.7 A total of 59.5% of patients with T2DM were having hypertension as comorbidity and 21.7% had neuropathy.
These findings were supported by previous studies indicating glimepiride and metformin FDC therapy is the most preferred choice of treatment in patients with diabetes-related complications and comorbid conditions for blood-glucose control and to reduce cardiovascular event risk.8,9,21 Early initiation of glimepiride and metformin FDC therapy may prevent progression of the disease as well as diabetes-related micro- and macrovascular complications and could provide legacy effect in the management of T2DM.10
A real-world analysis reported that glimepiride and metformin FDC was the preferred choice of treatment for T2DM patients with comorbidities and complications, for optimal blood glucose control.8 Despite several classes of antidiabetic drugs available in the market, glimepiride and metformin FDC was the most frequently prescribed in patients with hypertension and diabetes.9,22 In the current study, 86.7% of patients received glimepiride and metformin FDC as first-line therapy. This indicates that early initiation of low-dose glimepiride and metformin FDC therapy will achieve glycemic goals earlier.
The mean change in HbA1c levels was 1.2% at the end of the 6 months duration while FPG
and PPG levels were significantly reduced by 36.5 mg/dL and 50.2 mg/dL post-treatment, respectively. Our results were in concurrence with the study conducted by George J, who evaluated the efficacy of low-dose (0.5 mg) glimepiride and metformin FDC therapy in 941 patients with an early-stage T2DM.
A similar reduction in FPG (baseline: 151 mg/dL and after 3 months: 114 mg/dL), and PPG (baseline:
215 mg/dL, after 3 months: 158 mg/dL) levels were observed with no incidence of hypoglycemia and weight gain.14
Another study on low-dose glimepiride (0.5 mg) and metformin FDC conducted in patients with T2DM showed that this combination is more beneficial for young adults and patients with early-stage T2DM.15
In young adult patients (<40 years), FPG and PPG levels were reduced by 25% and 43%, respectively. Similarly, in patients with early-stage T2DM, FPG and PPG levels were reduced by 26% and 39%, respectively.15
In the present study, hypoglycemic events were reported in 7.7% of patients while 9.5% of patients recorded weight gain. The rate of hypoglycemic occurrence and weight gain were comparable with existing literature.11,23
Glimepiride at a low-dose (0.5 mg) is a more effective treatment due to its peripheral insulin-sensitizing nature. The down-regulation of insulin receptors caused by this agent may prevent hyperinsulinemia and ß-cell function failure.24 Overall results indicated good efficacy and tolerability in terms of achieving glycemic target and compliance to the treatment.
One of the key limitations of this study is the retrospective collection of data which limits the strength of the inference. Missing data of a few patients as a result of underreporting have compromised the analysis strength of the study outcomes. Large-scale, prospective, studies with longer follow-ups are necessary to validate these observations and will aid in further understanding of the clinical effectiveness and safety of glimepiride and metformin FDC in the management of T2DM.
Conclusion
A low dose of glimepiride (0.5 mg) and metformin (500/1000 mg) FDC therapy was found to be effective in achieving glycemic control through lowered HbA1c, FPG and PPG levels. The overall study indicated good tolerability with a low risk of hypoglycemia and weight gain. Thus, early initiation of low-dose (0.5 mg) glimepiride and metformin FDC is a promising approach in the management of T2DM.
Acknowledgments
We acknowledge Mr A Thamburaj and Ms Shashikala Borhade from USV Pvt. Ltd. for his assistance in the conduct of the project. The medical writing support was provided by Snehal Khanolkar from Sqarona Medical Communications LLP.
Contributors
RK Khinsvera, Tavish Arora, Harish Chandra Mishra, RP Chaubey, Yogesh Mehrotra, Manoj Agrawal, Anuj Kumar Saha, Kailash Narayan Singhal, Suresh Khatod, Awadhesh Kumar, Utpal Kant Biswas, Satendra Sharma, Paras Mal Jain, MP Khatri, Bhanwar Lal Bissu, MP Gupta, Anil Bhargava, Arvind Kumar, Deo Shankar Mishra, Ganpat Gehlot, Himanshu Mehta, K Shringi, Minesh Agarwal, Mukesh Agrawal, PK Singh, Pankaj Jain, Rajendra Kumar Chawda, SP Singh, Vijay Kumar Aggarwal, Vijay Shankar Upadhyay, Anurag Gupta, Abhay Jain, Hema Singh, Surendra Kumar Bhatter, Arvind Singh, Vinod Kumar Sahu, Anurag Srivastava, Rupesh Modi, Sanjay Gujrati, Manoj Kumar Sharma, Amit Kumar Das, Ankur Singh Gehlot, Girja Shanker Sinha, KP Joshi, Madhup Bakshi, Prem Prakash Patidar, Rajeev Kasliwal, Vinay Kumar Singh, Savita Agarwal, Ashish Modi, Srimant Kumar Sahu, Sanjay Teotia, Ashutosh Saxena, Sandeep Jain, Tagat Singh Rathore, Anand Malviya, Ajeet Singh Kothari, Amit Maheshwari, Manmohan Sharma, Vishwajeet Bembi, Yogesh Yadav, Manoj Kabra, Hafiz Noor Ali, Yogesh Sahni, Shailendra Kumar Singh, Madan Pal Singh, Rajeev Ranjan, Amit Kumar Sinha, Harshpreet Singh, Dau Lal Binani, Sandeep Singh, Dinesh Lalwani, Ajay Shah, Rajendra Pratap Singh, Sumit Kumar, Binay Prasad, Ashok Kumar, Gautam Bhandari, Navneet Kumar Maheshwari, Tarun Singhal, Manish Bansal, Abhay Kumar Sinha, Vijay Chawla, Arun Agarwal, Anish Jain, Shailesh Srivastava, Atul Khanna, Biplab Bandhopadhyay, Jitender Soni, Lalit Rathi, Sandeep Bhatnagar, Vrin Kumar Bhardwaj, Rajesh Singhal, Lalbahadur Singh Choudhary, Mritunjay Kumar Sinha, Rakesh Kumar Singh, Veerendra Singh, Vishwas Singh, Sunil Kumar, Jay Chordia, Prabhat Pandey, Kanhaiya Prasad Gaur, Ankur Sinha, Dawood Ansari, Manoj Gupta Jaiswal, Rajeev Kumar Mishra, Umesh Chandra, Monica Gupta, Kedar Chand Jindal, Dr Pankaj Jaisawal, UP Singh, SK Musaddi
Source of funding: The study was funded by USV Pvt. Ltd., Mumbai, India.
Conflict of interest: Dr Ashish Prasad and Dr Mayuri Talathi are employees of USV Pvt. Ltd. Dr Mahesh Abhyankar was an employee of USV Pvt. Ltd. during the conduct of the study. All other authors have no conflict of interest to declare.
References
- International Diabetes Federation. IDF Diabetes Atlas. 10th Edition, 2021. Available from: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf . Accessed February 08, 2021.
- Khunti K, Seidu S. Therapeutic inertia and the legacy of dysglycemia on the microvascular and macrovascular complications of diabetes. Diabetes Care. 2019;42(3):349-51.
- Feingold KR. Oral and injectable (non-insulin) pharmacological agents for the treatment of type 2 diabetes. 2021 Aug 28. In: Feingold KR, Anawalt B, Boyce A,
Chrousos G, de Herder WW, Dhatariya K, et al (Eds.). Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–.
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2017;40(Suppl 1):S64-S74.
- International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104(1):1-52.
- McGuire H, Longson D, Adler A, Farmer A, Lewin I; Guideline Development Group. Management of type 2 diabetes in adults: summary of updated NICE guidance. BMJ. 2016;353: i1575.
- Chawla A, Chawla R, Jaggi S. Microvascular
and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab. 2016;20(4):546-51.
- Unnikrishnan AG, Pandit K, George J, Venkataraman S, Abhyankar MV. Clinical utilization pattern of multiple strengths of glimepiride and metformin fixed dose combinations in Indian type 2 diabetes patients. J Assoc Physicians India. 2020;68(7):57-61.
- Sahay RK, Mittal V, Gopal GR, Kota S, Goyal G, Abhyankar M, et al. Glimepiride and metformin combinations in diabetes comorbidities and complications: real-world evidence. Cureus. 2020;12(9):e10700.
- Kim HS, Kim DM, Cha BS, Park TS, Kim KA, Kim DL, et al. Efficacy of glimepiride/metformin fixed-dose combination vs metformin uptitration in type 2 diabetic patients inadequately controlled on low-dose metformin monotherapy: a randomized, open label, parallel group, multicenter study in Korea. J Diabetes Investig. 2014;5(6): 701-8.
- Devarajan TV, Venkataraman S, Kandasamy N, Oomman A, Boorugu HK, Karuppiah SKP, et al. Comparative evaluation of safety and efficacy of glimepiride and sitagliptin in combination with metformin in patients with type 2 diabetes mellitus: Indian multicentric randomized trial - START Study. Indian J Endocrinol Metab. 2017;21(5):745-50.
- National Collaborating Centre for Chronic Conditions (UK). Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update). London: Royal College of Physicians (UK); 2008.
- Singh SK. Commentary on “consensus recommendations on sulfonylurea and sulfonylurea combinations in the management of type 2 diabetes mellitus: International Task Force”. Indian J Endocrinol Metab. 2018;22(1):158-9.
- George J. Starting with low dose sulfonylurea and metformin in early stage type 2 diabetes mellitus. Indian J Endocrinol Metab. 2015;19(2):309.
- Banset A, Mahato B, Shrestha BL, Bhattarai L, Shrestha MR, Shah RDP, et al. Efficacy and safety of low dose glimepiride - metformin fixed dose combination [0.5 mg
glimepiride + 500 mg metformin sustained release (SR)] in patients with type 2 diabetes mellitus (T2DM) in Nepal. Indian Medical Gazette. 2019;CLIII:94-8.
- Urakaze M, Yamazaki K, Usui I, Iwata M, Uno T, Murakami S, et al. Glimepiride (0.5 mg/day) administration improves glycemic control without weight gain in Japanese type 2 diabetic patients. J Japan Diab Soc. 2007;50(12):835-41.
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):
S111-24.
- Chawla R, Madhu SV, Makkar BM, Ghosh S, Saboo B, Kalra S; RSSDI-ESI Consensus Group. RSSDI-ESI clinical practice recommendations for the management of type 2 diabetes mellitus 2020. Indian J Endocrinol Metab. 2020; 24(1):1-122.
- Yoon KH, Shin JA, Kwon HS, Lee SH, Min KW, Ahn YB, et al. Comparison of the efficacy of glimepiride, metformin, and rosiglitazone monotherapy in Korean drug-naïve type 2 diabetic patients: the practical evidence of antidiabetic monotherapy study. Diabetes Metab J. 2011;35(1):26-33.
- Melikian C, White TJ, Vanderplas A, Dezii CM, Chang E. Adherence to oral antidiabetic therapy in a managed care organization: a comparison of monotherapy, combination therapy, and fixed-dose combination therapy. Clin Ther. 2002;24(3):460-7.
- Prasanna Kumar KM, Seshadri K, Aravind SR, Deb P, Modi KD, Gopal RA, et al. Real-world observational study of glimepiride and metformin fixed-dose combination along with insulin in the management of type 2 diabetes mellitus: Indian experience. Cureus. 2021;13(1):e13020.
- Tripathi S, Tiwaskar M, Kota S, Parthan G, Dasgupta A, Mohanasundaram S, et al. Need of single pill fixed-dose combination with glimepiride in management of diabetes mellitus. J Assoc Physicians India. 2019;S1:30-3.
- Abd Al-Aziz MF, Abu Shady M, Abd El-Hamid S. Safety and efficacy of fixed dose combination of glimepiride and metformin in type 2 diabetic patients in Egypt: a real-life study. Medical J Cairo Univ. 2018;86(5):2599-603.
- Bermúdez-Pirela VJ, Cano C, Medina MT, Souki A, Lemus MA, Leal EM, et al. Metformin plus low-dose glimeperide significantly improves Homeostasis Model Assessment for insulin resistance (HOMA(IR)) and beta-cell function (HOMA(beta-cell)) without hyperinsulinemia in patients with type 2 diabetes mellitus. Am J Ther. 2007;14(2): 194-202.