Abstract
Background and objective: Chronic kidney disease (CKD), and its increasing global burden, is associated with significant morbidity and mortality. This survey-based study aims to capture the knowledge, attitude and practices (KAP) amongst practicing physicians in considering sodium-glucose cotransporter 2 inhibitors (SGLT2i) for the prevention and progression of CKD in diabetic or nondiabetic individuals. Methodology: An online questionnaire-based survey was conducted among 262 health care practitioners (HCPs) who manage people with CKD with or without diabetes. The survey was prepared as a Google form and circulated through email to different HCPs. The survey consisted of 6 knowledge-based questions, 4 attitude-based questions and 4 practice-based questions. The forms were filled up voluntarily by the participants and the authors had no control over the response provided. All the responses were consolidated using Microsoft Excel and analyzed. Results: A total of 262 HCPs from different regions of the country participated in the survey. About 87% to 94% of the participants were aware that SGLT2i, specifically dapagliflozin, is approved for use in CKD patients with or without diabetes. About three-fourths of the HCPs accepted that an initial drop in estimated glomerular filtration rate (eGFR) occurs upon initiation of dapagliflozin treatment. Almost 90% of them acknowledged the importance of screening for CKD in diabetic patients, and the majority were aware of the renal benefits of SGLT2i. Almost 96% of HCPs consider that dapagliflozin could be used in all patients with CKD irrespective of their diabetes status. Major determining factors with respect to a setback in practice are fear of side effects (54%) and hesitation in switching to newer medications when older medications work fine (34%). Conclusion: SGLT2i have demonstrated significant clinical benefits in patients with CKD with or without diabetes. This survey has shown good awareness among clinicians of the beneficial role of SGLT2i in CKD.
Keywords: KAP, health care practitioners, survey, diabetic kidney disease, SGLT2i, diabetes, dapagliflozin
Introduction
Chronic kidney disease (CKD), and its increasing global burden, is associated with significant morbidity and mortality.1 In addition to the distressing effects of decreased renal function, the treatment burden also severely impaired the quality of life of affected individuals.2,3 With the alarming global prevalence of comorbidities, including diabetes, hypertension and obesity, there is a corresponding significant surge in the prevalence of CKD.4,5 Diabetic kidney disease coexisting in 30% and 40% of individuals with type 1 and type 2 diabetes (T2DM) remains a grave concern, highlighting the need to prevent CKD and its progression.6
There are no new medicines that have shown to decelerate the progression of CKD to kidney failure in the last two decades. Surprisingly, sodium-glucose cotransporter 2 inhibitors (SGLT2i) came out as reno-protective agents due to their potential to treat diabetes and kidney dysfunction.7,8 Various clinical trials and consensus guidelines have recommended the use of SGLT2i in individuals with T2DM to reduce risks for CKD and cardiovascular disease (CVD).9-12 The mechanisms of nephroprotective action of SGLT2i extend beyond glucose-lowering, weight-lowering and blood pressure-lowering effects that accompany their glucosuric action in people with diabetes. They protect the glomeruli by reducing intraglomerular pressure, improving tubular oxygenation and metabolism and reduction in renal inflammation and fibrosis.
Among the class of SGLT2i, dapagliflozin is the first drug to be approved by the Food and Drug Administration (FDA) in April 2021 for use in CKD patients irrespective of their diabetes status.13
The results of DECLARE-TIMI 58 suggested a role for SGLT2i in the prevention of diabetic kidney disease. Dapagliflozin had demonstrated a favorable effect on urinary albumin-to-creatinine ratio (UACR) and renal-specific outcome across baseline UACR categories, including patients with normal albumin excretion.14
In 2020, generic and affordable versions of dapagliflozin were made available by various manufacturers. Despite supporting evidence and improved affordability, the usage of dapagliflozin is still inadequate by the Indian practitioners who manage CKD based on treatment audit.9
Experts certainly follow guidelines based on high-quality evidence from landmark studies. However, when it comes to the practical application of the guidance, they must go through certain adaptations that consider the newer evidence and clinical practice guidelines and patient’s context, such as comorbidities, economic status, education status and ability to comply with the proposed management. So, the real-world usage or complete adherence to guidelines may not be exactly reflective of a randomized clinical trial setting. Ultimately, real-world usage is based on evidence, expert guidelines and experience-based applications.
Despite all the affirmative findings and guideline statements and regulatory approvals, continuing medical education (CME) programs, the question remains to what extent the physicians are really applying this knowledge. If this knowledge does not translate into practice, it undermines all the efforts and the actual benefits are never reaped. Therefore, the current survey was conducted to capture the knowledge, attitude and practices (KAP) amongst practicing physicians in considering SGLT2i for the prevention and progression of CKD in diabetic or nondiabetic individuals. Through this study, the authors sought to identify and highlight the gaps and eventually hope that the practitioners would reduce these gaps and pass on the benefits of SGLT2i based on recent evidence and guideline recommendations in the management of CKD.
Materials and Methods
An online questionnaire-based survey was conducted among 262 health care practitioners (HCPs) who manage people with CKD with or without diabetes. The HCPs practicing in different geographical locations were selected based on random sampling method.
The survey was prepared as a Google form and circulated through email to different HCPs. The survey consisted of 6 knowledge-based questions, 4 attitude-based questions and 4 practice-based questions.
The forms were filled out voluntarily by the participants who had no control over the responses provided. All the responses were consolidated using Microsoft Excel and analyzed.
Results
A total of 262 HCPs from different regions of the country participated in the survey.
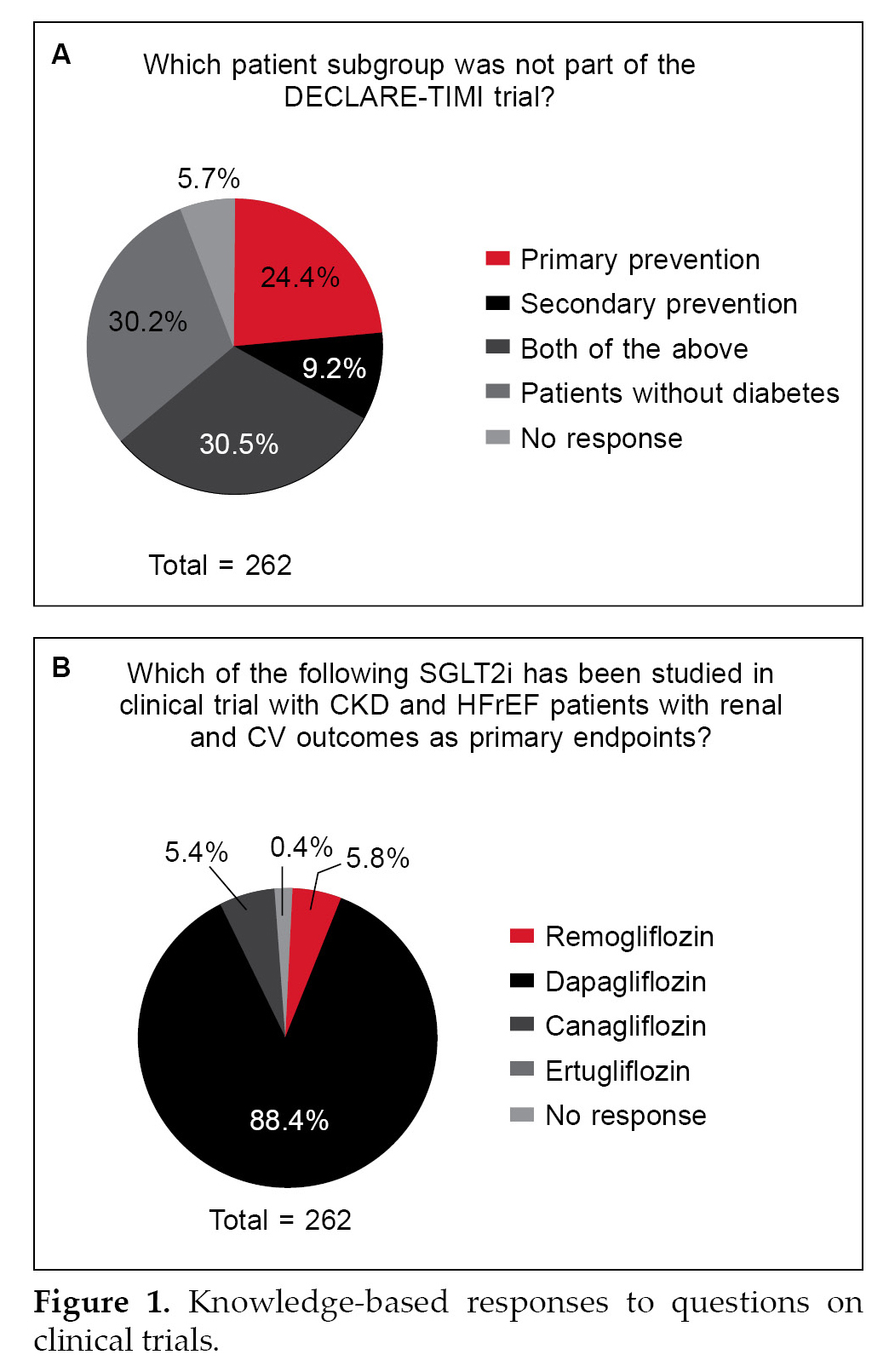
Two-third of the HCPs were correct in their responses of the patient group included in the DECLARE-TIMI trial (Fig. 1A) and almost 90% were aware that dapagliflozin was the drug studied in the clinical trial including patients with CKD and heart failure with reduced ejection fraction (HFrEF) (Fig. 1B).
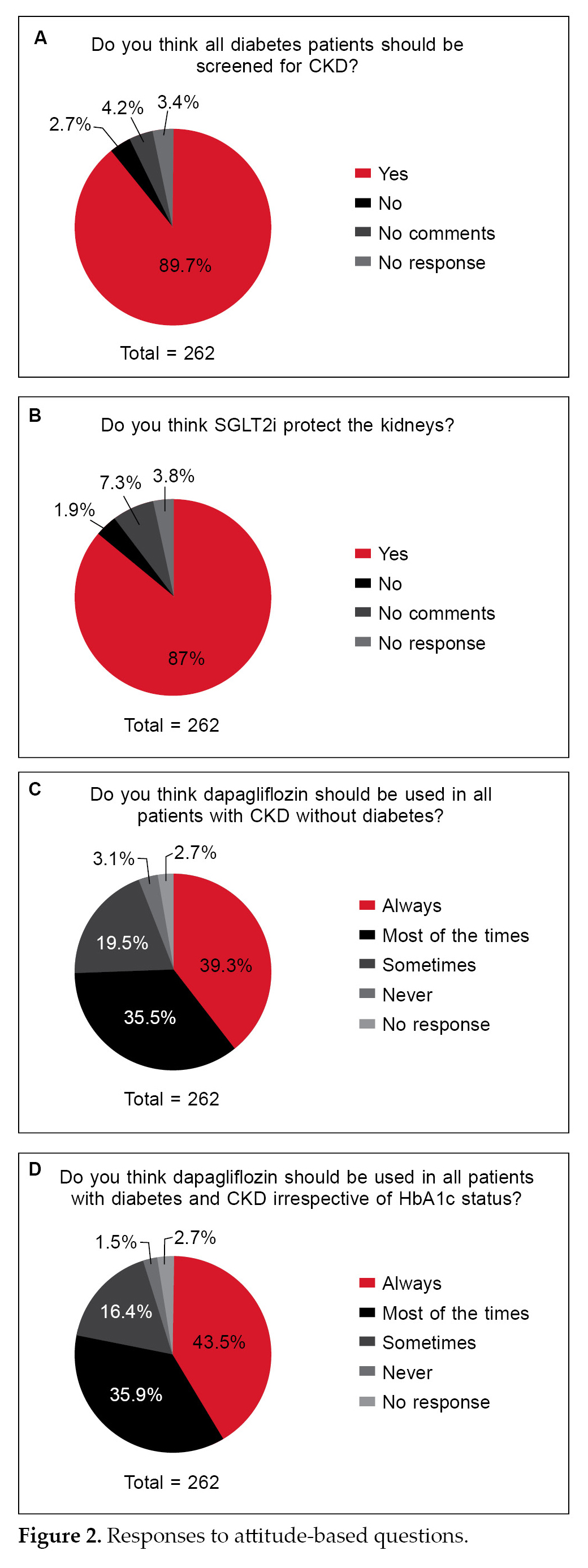
About 87% to 94% of the participants were aware that SGLT2i, specifically dapagliflozin, is approved for use in CKD patients with or without diabetes and is considered a first-line treatment choice for diabetes in patients with diabetes and CKD (Table 1). About three-fourths of the HCP accepted the initial drop of estimated glomerular filtration rate (eGFR) upon initiation of dapagliflozin treatment.
Almost 90% of the HCPs acknowledged the importance of screening for CKD in diabetic patients, and the majority were aware of the renal benefits of SGLT2i. Three-fourths of the respondents were aware about the use of dapagliflozin in CKD patients irrespective of the glycated hemoglobin (HbA1c) status.
|
Table 1. Responses to Knowledge-based Questions
|
|
Questions to map the knowledge gaps
|
No. of responses
|
True/Yes
|
|
SGLT2i are recently approved for the management of CKD with or without diabetes.
|
245
|
94.3%
|
|
Dapagliflozin reduced the long-term decline of eGFR?
|
245
|
92.2%
|
|
In patients with CKD, SGLT2i is indicated as first-line agent of T2DM with CKD.
|
241
|
87.1%
|
|
There will be an initial drop of eGFR upon initiation of dapagliflozin. Is it acceptable?
|
242
|
74%
|
Majority of the HCPs opined that people with diabetes must be screened for CKD and consider SGLT2i for management (Fig. 2A and 2B). Almost 96% of HCPs consider that dapagliflozin could be used for management in all patients with CKD irrespective of their diabetes status (Fig. 2C and 2D).
Practice-based Questions
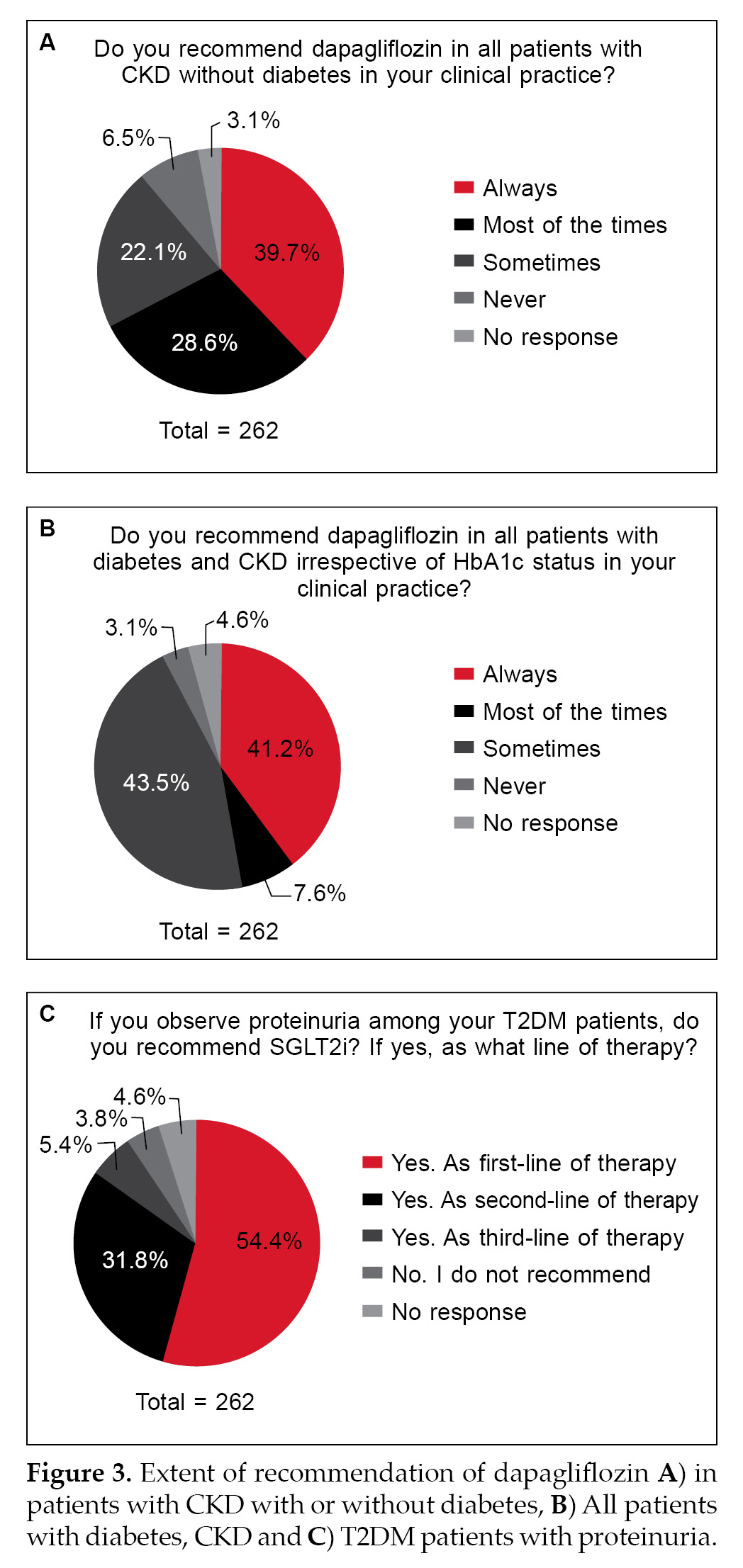
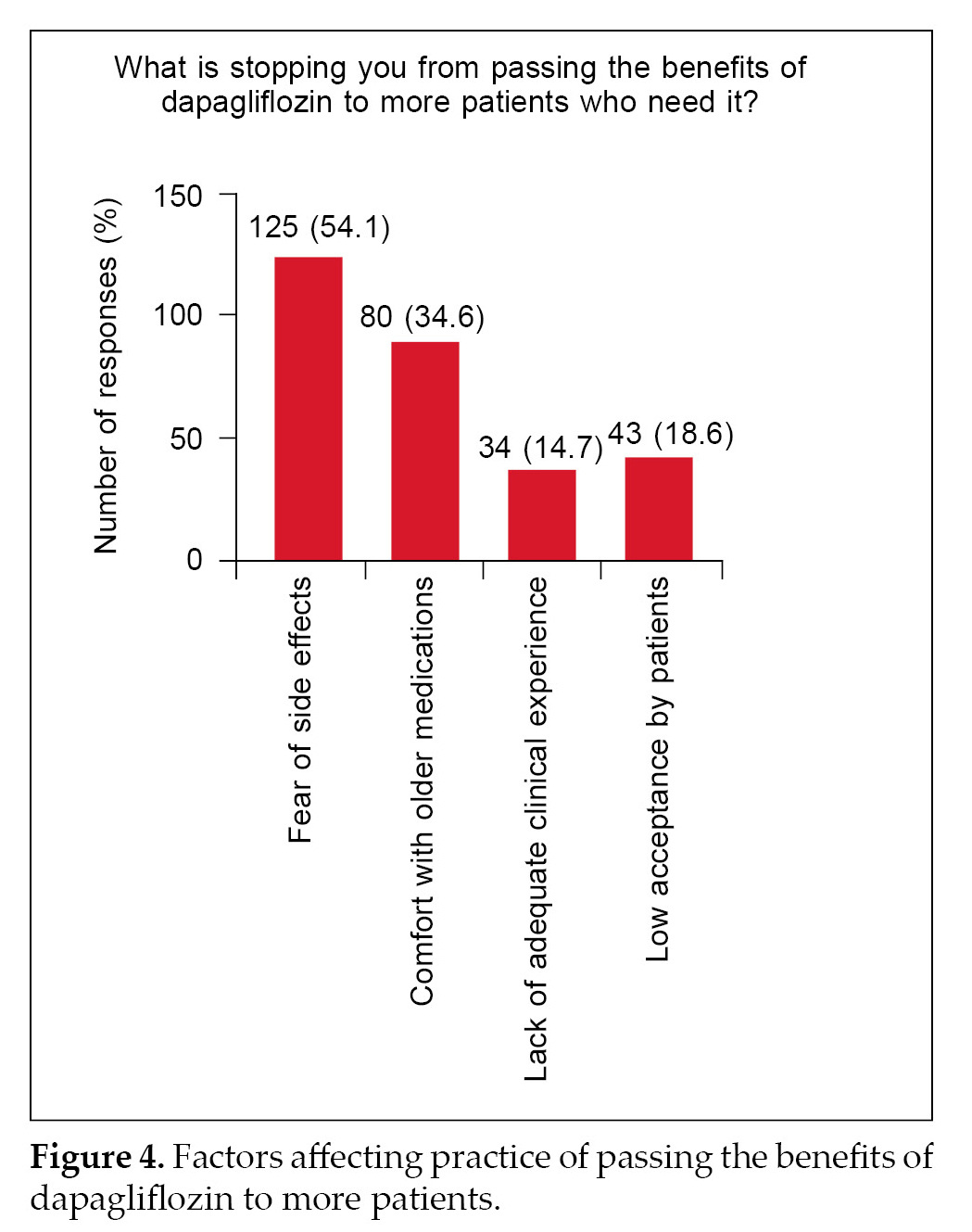
Only about 40% to 50% of the respondents always recommended the use of SGLT2i in patients with proteinuria or dapagliflozin for CKD patients with orwithout diabetes (Fig. 3A-3C). Major determining factors with respect to a setback in practice are fear of side effects (54%) and hesitation in switching to newer medications when older medications work fine
(34%) (Fig. 4).
Discussion
Knowledge among HCPs about SGLT2i and the efficacy of dapagliflozin to prevent the decline of eGFR is noteworthy (Fig. 1). Based on the responses to different types of questions, it was seen that there is a high level of awareness on the pertinent aspects on the usage of SGLT2i for the management of people with CKD. Various clinical trials and consensus guidelines have recommended the use of SGLT2i in individuals with T2DM to reduce the risks of CKD and CVD.9 The KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease recommends the administration of SGLT2i in patients with T2DM and CKD with an eGFR ≥20 mL/min/1.73 m2.10 However, for people with type 2 diabetes and diabetic kidney disease, use of an SGLT2i in individuals with eGFR ≥20 mL/min/1.73 m2 and UACR ≥200 mg/g creatinine is recommended to reduce CKD progression and cardiovascular events. This is a change in eGFR from previous recommendations that suggested an eGFR level ≥25 mL/min/1.73 m2.11 Among SGLT2i, dapagliflozin was the first drug to be approved by the FDA in April 2021 for use in CKD patients irrespective of their diabetes status based on the findings of the DAPA-CKD trial.12 NICE has also recommended dapagliflozin as a treatment option for certain people with CKD in adults.13 Dapagliflozin was found to reduce the progression of kidney disease in the DECLARE-TIMI 58 trial, which included patients with T2DM and either known CVD (secondary prevention cohort) or at least two risk factors for CVD (primary prevention cohort). Most of the patients in DECLARE-TIMI 58 had preserved renal function.14
More than 90% of the HCP could recognize dapagliflozin as the SGLT2i with approved usage.
A critical observation from various trials is an initial decline in eGFR upon initiation of treatment with SGLT2i. These drugs inhibit sodium and glucose reabsorption in the proximal tubule increasing sodium and chloride delivery to the macula densa. This results in afferent arteriolar vasoconstriction, causing a reduction in the intraglomerular pressure and the GFR.15,16 The question pertaining to the decline in initial eGFR rate upon initiation of SLGT2i was correctly answered by three-fourths of the practitioners. Hence, the knowledge about SGLT2i in cardiovascular outcome trial (CVOT) and renal outcomes are considered to be very good. Aligning with the knowledge-based responses, the attitude of the doctors towards considering SGLT2i, especially the approved dapagliflozin for CKD patients with or without diabetes, is gratifying (Fig. 2). The majority of practitioners believe that dapagliflozin may help in preventing the long-term decline in eGFR.
When it comes to practice, only less than 50% always consider SGLT2i or dapagliflozin as a choice of treatment in CKD individuals or those with proteinuria (Fig. 3). This is concerning despite the fact that several clinical trials and regulatory agencies have approved the drug as a first-line treatment option in CKD patients.8,13 Fear of side effects was the first major concern (Fig. 4). SGLT2i are known for their side effects of urinary tract infections, among others.17,18 The answers have two limitations: 1) Did the responders have major concerns regarding the incidence of genital mycotic infections (GMI) and other potential side effects like increased hypoglycemia, among others? 2) The questionnaire itself does not lend any scope to understanding the specific concern of side effects. Further, we also cannot infer whether this fear of side effects is fully qualified or is it more of a perception issue. Future studies with specific questions may throw clarity on this aspect.
The other point that stops the prescribers from passing on the benefits of dapagliflozin to more patients, as conveyed by 34.6% of respondents, is comfort with old medications (Fig. 4). This response also has a few limitations as regards which medication and what do they mean by discomfort. We do not know whether this is due to fear of hypoglycemia or any possible drug interactions with other medicines.19,20 These two aspects, i.e., fear of side effects and comfort with old medications, are eminently addressable with data and studies and concerted education efforts.
Summary
SGLT2i have demonstrated significant clinical benefits in patients with CKD with or without diabetes.
This survey has shown good awareness among clinicians of the beneficial role of SGLT2i in CKD. However, the usage and attitude towards SGLT2 in the management of patients with CKD are low, and there could be several reasons for this. Overcoming these challenges will help in the broader usage of SGLT2i in people with CKD, which may help in improving outcomes in such patients.
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709-33.
- Al-Mansouri A, Al-Ali FS, Hamad AI, Mohamed Ibrahim MI, Kheir N, Ibrahim R, et al. Assessment of treatment burden and its impact on quality of life in dialysis-dependent and pre-dialysis chronic kidney disease patients. Res Social Adm Pharm. 2021;17(11):1937-44.
- Kefale B, Alebachew M, Tadesse Y, Engidawork E. Quality of life and its predictors among patients with chronic kidney disease: a hospital-based cross sectional study. PLoS One. 2019;14(2):e0212184.
- Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316(6):602-10.
- Navaneethan SD, Zoungas S, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, et al. Diabetes management in chronic kidney disease: synopsis of the 2020 KDIGO Clinical Practice Guideline. Ann Intern Med. 2021;174(3):385-94.
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032-45.
- Li N, Lv D, Zhu X, Wei P, Gui Y, Liu S, et al. Effects of SGLT2 inhibitors on renal outcomes in patients with chronic kidney disease: a meta-analysis. Front Med (Lausanne). 2021;8:728089.
- Tuttle KR, Brosius FC 3rd, Cavender MA, Fioretto P, Fowler KJ, Heerspink HJL, et al. SGLT2 inhibition for CKD and cardiovascular disease in type 2 diabetes: report of a Scientific Workshop sponsored by the National Kidney Foundation. Am J Kidney Dis. 2021;77(1):94-109.
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-46.
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022;102(5S):S1-S127.
- ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al; American Diabetes Association. 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S191-S202.
- US Food and Drug Administration. FDA approves treatment for chronic kidney disease. April 30, 2021. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-chronic-kidney-disease
- NICE recommends dapagliflozin for people with chronic kidney disease. November 5, 2021. Available from: https://www.nice.org.uk/news/article/nice-recommend-dapagliflozin-for-people-with-chronic-kidney-disease#
- Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606-17.
- Bakris G, Oshima M, Mahaffey KW, Agarwal R, Cannon CP, Capuano G, et al. Effects of canagliflozin in patients with baseline eGFR <30 ml/min per 1.73 m2: subgroup analysis of the randomized CREDENCE trial. Clin J Am Soc Nephrol. 2020;15(12):1705-14.
- Oshima M, Jardine MJ, Agarwal R, Bakris G, Cannon CP,Charytan DM, et al. Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int. 2021;99(4):999-1009.
- Halimi S, Vergès B. Adverse effects and safety of SGLT-2 inhibitors. Diabetes Metab. 2014;40(6 Suppl 1):28-34.
- Singh M, Kumar A. Risks associated with SGLT2 inhibitors: an overview. Curr Drug Saf. 2018;13(2):84-91.
- Lam D, Shaikh A. Real-life prescribing of SGLT2 inhibitors: how to handle the other medications, including glucose-lowering drugs and diuretics. Kidney360. 2021;2(4):742-6.
- Tian B, Deng Y, Cai Y, Han M, Xu G. Efficacy and safety of combination therapy with sodium-glucose cotransporter 2
inhibitors and renin-angiotensin system blockers in patients with type 2 diabetes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2022;37(4):720-9.