Abstract
It is a well-known fact that the knowledge of their current glucose readings empowers people with diabetes to evaluate and monitor the trends in glucose fluctuations and take informed decisions on adjusting their medicines, food intake, and physical activity. Glucose monitoring technology has undergone a technological evolution and has improved diabetes care in patients living with type 2 diabetes. This has also made the need to efficiently and effectively utilize blood glucose monitoring tools. Glucometric checklists offer a standardized approach to glucometric guardianship which is necessary to improve the process of drug choice and dose titration. The stepwise factors included in the glucometric guardianship checklist include procurement, distribution, pre-testing hygiene, testing, recording, action, disposal, quality control, and procedure safety. This article has reviewed the significance of glucometric guardianship and has also developed checklists for efficient glycemic management.
Keywords: Type 2 diabetes, glucometric guardianship, checklist, glucose meters, glycemic triad.
Introduction
Introduced in the late 1970s and regulatory clearance received for the first time in 1980, blood glucose monitoring (BGM) has revolutionized the self-care of people with diabetes. A knowledge of their current glucose readings empowers people with diabetes to assess and better understand their glucose patterns to adjust their food intake, activity and medications to achieve their glycemic goals.1
BGM is an essential part of case management in patients with diabetes. Having very high or very low blood glucose levels may affect cellular function and could be life-threatening, including direct health care costs and reduced productivity; if not managed appropriately. It serves as a critical measure in individuals with ongoing diabetes management.2
The American Diabetes Association (ADA) 2017 reported that the total estimated cost of diagnosed diabetes in 2017 was $327 billion;3 however, the direct cost of treating complications, including hospitalizations, emergency room visits and nondiabetes prescriptions, along with indirect costs related to lost/reduced productivity and human costs account for almost 73% of the total diabetes cost.4
The need to effectively and efficiently utilize BGM tools and resources to improve diabetes outcomes is indisputable. Continuous glucose monitoring is set to bring a fundamental change in the treatment of diabetes and patient engagement of those affected with this disease.5 Over the years, diabetes practice has become more and more algorithm-based and statistic oriented, which facilitates the patient-centric treatment approach. Glucocentric screening and monitoring, added to this, have led to the neglect of a holistic medicine approach.6 Hence, in this review, we have reviewed the significance and value of glucometric guardianship. We have also attempted to design checklists to facilitate routine clinical practice and impact decision-making.
Glycemic Guardianship
Kalra et al proposed the concept of glycemic guardianship, which was defined as “activities carried out by the health care team and health care system to ensure optimal care of the person, or group of peoples, living with diabetes.” Glycemic guardianship is a novel concept that can be functional at the national/regional level as well as the individual level and is ideally considered in partnership with individuals living with diabetes. The World Health Organization’s Global Diabetes Compact (GDC) targets provide an umbrella for all activities related to glycemic guardianship.7
GDC emphasizes five targets comprising diagnosis of diabetes in 80% of individuals living with diabetes, achieving glucometric optimization in 80% of individuals diagnosed with diabetes, blood pressure control in 80% of individuals diagnosed with diabetes, ensuring statin prescription in 60% of individuals with diabetes who are 40 years or more in age, availability of affordable insulin, and blood glucose self-monitoring for all the people with type 1 diabetes. With the second-largest population of diabetes individuals living in India, the country’s health care system and providers must strive to screen, diagnose, manage and prevent diabetes and related complications. While the prevalence of diabetes has increased, so has the proportion of those living with undiagnosed diabetes, thereby diminishing or counterbalancing the advances in diabetes care and delivery.7
With the Indian pharmaceutical industry being the world leader in manufacturing good quality drugs and devices, the easy availability of good quality and reasonably priced glucose monitoring devices and ancillaries has also been facilitated. With this, glucovigilance and personalized diabetes management have become integral to diabetes management and care.8
The Domains of Glucometric Guardianship
The benefits of glucometric guardianship are that it encompasses the physical and electronic infrastructure and further delineates the roles and responsibilities of various health care team members. The infrastructural requirements of glucometric guardianship include hardware (glucose measuring devices and ancillary supplies) and software (data recording and analysis). Table 1 shows the domains of glucometric guardianship.
|
Table 1. Infrastructure of Glucometric Guardianship
|
|
Equipment
|
· Choice of the glucose monitoring device, e.g., Glucose meters vs. flash glucose monitoring device; glucose meters/FGMS model
· Individual device or common device: e.g., prefer individual glucose meters if expected hospital stay of >2-3 days or if the expected number of glucometer pricks is >20
· Glucose sticks: available at bedside/central station
· Lancets: available at bedside/central station
· Alcohol swabs: available at bedside/central station
· Meter calibration: needed/not needed: at what frequency
|
|
Roles and responsibilities
|
· Glucose monitoring: by-
· Data entry: by-
· Analysis: by-
· Disposal of used ancillary supplies: by-, at-
· Red flag range: e.g., call duty doctor if plasma glucose <70 mg/dL and >400 mg/dL; check urine/blood ketones if BG >400 mg/dL
· Treatment/titration: by-
· Meter calibration: by-
|
|
Patient-specific glucometric guardianship
|
· Frequency of monitoring
· Site of prick; rotation of fingers
· De-escalation of frequency of monitoring: e.g., if BG 100-200 mg/dL; <20% change in consecutive glucose values at the current frequency
· Escalation of frequency of monitoring: e.g., if BG <100 or >200 mg/dL; >20% change in consecutive glucose values
|
The advantages of glucometric guardianship are given in Table 2 given below.
Glucometric Guardianship Checklist
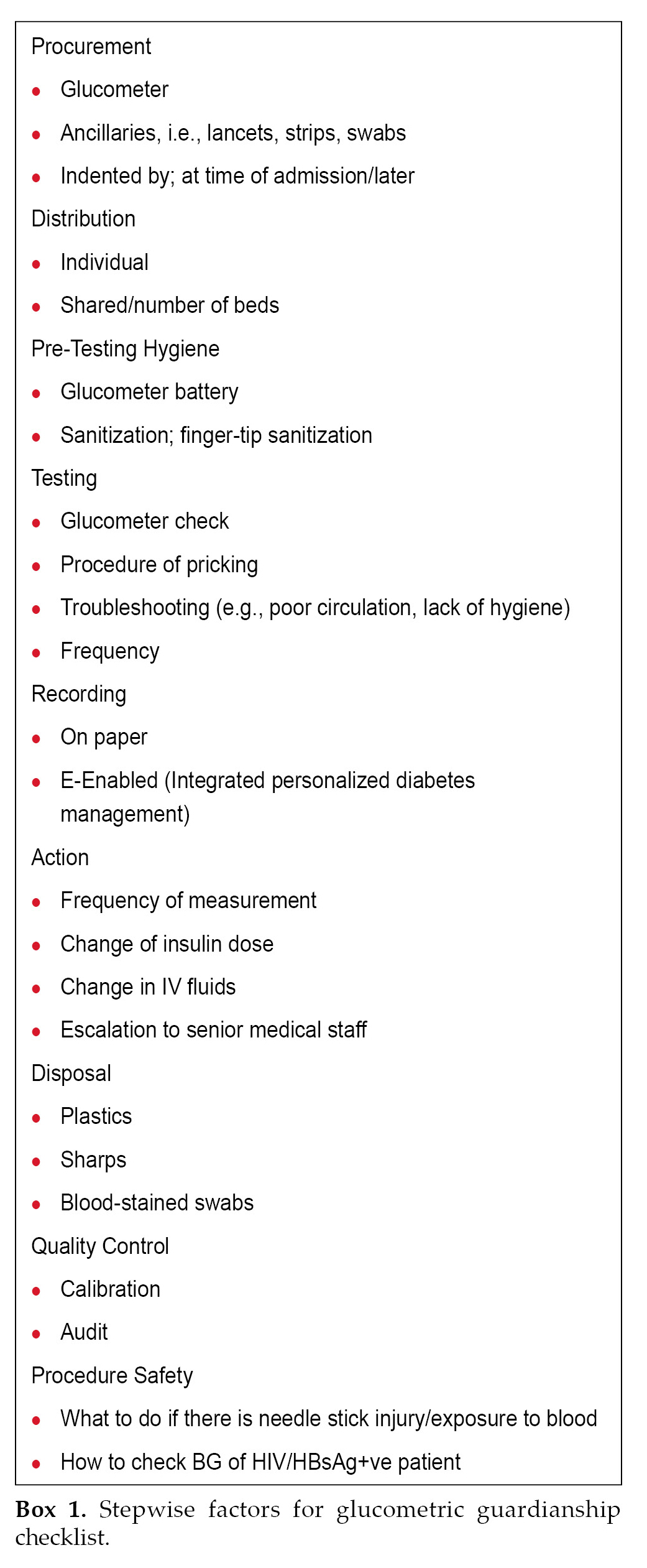
“You can’t improve what you can’t measure accurately” is an adage illustrating the dilemma facing attempts to optimize glycemic control. Glucometric guardianship ensures appropriate measurement and monitoring of glucose levels to ensure alertness in glycemic management and agility in anticipating and identifying suboptimal glycemic parameters and responding to them.9 (Box 1)
In diabetes care, several well-developed algorithms are available for glycemic management in the inpatient and outpatient settings; however, they do not integrate the nuances of glucose monitoring. Thus, glucometric measurements act as a challenge as well as a facilitator to achieving optimal glucose control. Hence, a standardization of glucometrics and adopting a practice-based approach to glucometric guardianship is essential to improve the process of drug choice and dose titration.9
The objective of developing these checklists are: (i) to emphasize the need for accurate measurement,
|
Table 2. Advantages of Glucometric Guardianship
|
|
Accurate determination of glucose control
Avoidance of hypo-/hyperglycemia
Prevention of complications
Facilitation of audit
Comparison and research
|
monitoring, of glucose levels to improve the management of diabetes; (ii) to facilitate the process of glucometric guardianship by outlining the steps and factors to consider when monitoring and analyzing blood glucose patterns in individuals with diabetes; (iii) standardize the process of glucose monitoring and ensure that health care providers have a systematic approach to managing blood glucose levels in different care settings.
Outpatient Glucose Monitoring
Glucose control is an imperative and essential component of outpatient deviations in blood glucose level care in diabetes. Clinical scenarios with better glucose control have been shown to improve patient outcomes. Glycated hemoglobin (HbA1c) can be used to assess the quality of outpatient glycemic control. Glucometrics has been shown to allow comparison of inpatient glycemic control among hospitals and patient care units and will allow institutions to evaluate the success of their quality improvement initiatives.10
The availability of point-of-care meters capable of storing glucose measurements from many patients eases, to some degree, the burden of data collection.11
Inpatient Glucose Monitoring
Inpatient hyperglycemia and hypoglycemia are related to worse patient outcomes, such as additional wound infections, prolonged hospital stays and higher mortality rates, especially in ICU. In most cases, an inpatient target glucose range of 140-180 mg/dL
may represent the optimal balance for avoiding complications associated with extraordinarily high- and low-glucose levels.12
Emergency/Casualty
Many patients reporting to emergency care could have hyperglycemia who may be undiagnosed. Uncontrolled hyperglycemia and iatrogenic hypoglycemia are associated with a broad range of adverse outcomes, with insulin commonly attributing to adverse drug events if the patient is a known case of diabetes on treatment. While insulin and hypoglycemia management protocols allow for managing patients in emergency care, there is a lack of glucometric standardization and limited resources acting as challenges in diabetes management.13
Checklists
Because of the challenges in managing outpatient, inpatient, and emergency patients, we have attempted to devise CHECKLISTS to test, monitor and analyze the blood glucose pattern in individuals with diabetes
|
Table 3. Checklist for Outpatients
|
|
Patient ID
|
Visit 1
|
Visit 2
|
|
Procurement:
if the patient using a glucometer (which brand)
of a meter (which brand if patient not using glucometer)
of ancillaries, i.e., lancets, strips, swabs (which brand)
|
Procurement
Which brand of glucometer
Recommended brand of glucometer
Recommended brand of ancillaries
Comments, if any
|
Cross check availability of
Glucometer
Ancillaries
|
|
Usage pattern and training:
Individual/shared/family
Training of
How to use the glucometer
Testing: change of lancet after how many pricks
How to share readings with the HCP
|
Individual£ Shared£ Family£
Done – Y£ N£
Done – Y£ N£
Done – Y£ N£
Comments, if any
|
Cross-check usage pattern and technique
|
|
Pre-testing hygiene:
Time/Date of calibration
Glucometer battery
Sanitization
a) Fingertip sanitization
b) Glucometer Disinfection
Needle
|
Time/Date
Glucometer
battery working Y£ N£
Sanitization Y£ N£
Done Y£ N£
Done Y£ N£
Needle Checked Y£ N£
Comments, if any
|
Time/Date
Glucometer
battery working Y£ N£
Sanitization Y£ N£
Done Y£ N£
Done Y£ N£
Needle Checked Y£ N£
Comments, if any
|
|
Testing:
Glucometer check
Confirm glucose units (mg or mmol)
Procedure of pricking/intensity of lancet prick
|
Done – Y£ N£
Done – Y£ N£
Checked – Y£ N£
|
Done – Y£ N£
Done – Y£ N£
Checked – Y£ N£
|
Cont'd
Cont'd
|
Table 3. Checklist for Outpatients
|
|
Patient ID
|
Visit 1
|
Visit 2
|
|
Troubleshooting frequency
|
Done – Y£ N£
If Y, specify the reason
Comments if any
|
Done – Y£ N£
If Y, specify the reason
Comments, if any
|
|
Frequency (Appendices 1 and 2)
|
|
|
|
Recording:
Cross-checking glucometer data with the CBG log
E-enabled [Integrated personalized diabetes management (IPDM)]
On paper
|
Done – Y£ N£
Y£ N£
Y£ N£
|
Done – Y£ N£
Y£ N£
Y£ N£
|
|
Action:
Change in diet/physical activity
Change in OAD
Change in insulin dose
|
Done – Y£ N£
If Y, Specify
Done – Y£ N£
If Y, Which OAD?
Done – Y£ N£
If Y Specify
|
Done – Y£ N£
If Y, Specify.
Done – Y£ N£
If Y, which OAD?
Done – Y£ N£
If Y Specify
|
|
Storage of strips
Disposal: (home/hospital)
Plastics
Sharps
Blood-stained swabs
|
Storage done as per instruction Y£ N£
Disposal: Home (Y) or Hospital (Y)
Done – Y£ N£
Done – Y£ N£
Done – Y£ N£
|
Cross-check storage and disposal
|
|
Appendix 1: Glucose Monitoring Log (Outpatients)
|
|
Date/Time
|
BB
|
AB
|
BL
|
AL
|
BD
|
AD
|
3 am
|
Comments
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Week: ….Date Onwards
|
Date/Time
|
BB
|
AB
|
BL
|
AL
|
BD
|
AD
|
3 am
|
Comments
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
BB, BL, BD: Before Breakfast, Lunch, Dinner
AB, AL, AD: After Breakfast, Lunch, Dinner
|
Appendix 2: Diet Log (Outpatients)
|
|
Day/Time
|
Diet log/changes in diet/activity/illness next to the blood glucose levels
|
|
Monday
|
|
|
Tuesday
|
|
|
Wednesday
|
|
|
Thursday
|
|
|
Friday
|
|
|
Saturday
|
|
|
Sunday
|
|
BB, BL, BD: Before Breakfast, Lunch, Dinner
AB, AL, AD: After Breakfast, Lunch, Dinner
|
Table 4. Checklist for Ward Patients
|
|
Ward ID
|
Audit No. 1
|
Audit No. 2 onwards
|
|
Procurement:
of meter (which brand?)
of ancillaries, i.e., lancets, strips, swabs (e.g., which brand)
|
Recommended brand of glucometer
Recommended brand of ancillaries
Comments, if any
|
CMEs and CNEs should be conducted regularly (monthly or quarterly). This should be accompanied/ followed by audits at frequent intervals.
These audits are targeted at ward nurses/diabetes educators. It is expected that these health care providers will disseminate the right knowledge to all patients admitted to their ward as well as their caregivers.
|
|
Usage pattern:
Individual
shared/beds
|
Individual£
Shared£
|
|
Pre-testing practices:
Glucometer battery
Sanitization
a) Fingertip sanitization
b) Glucometer disinfection
Setting intensity of lancet prick
|
Glucometer battery working – Y£ N£
Sanitization
Done Y£ N£
Done Y£ N£
Done as per skin thickness over the fingertip comments, if any
|
|
Testing:
Glucometer check
Confirm glucose units (mg or mmol)
Procedure of pricking
Loading the lancet
Rotating site of finger prick
Troubleshooting (poor circulation, lack of hygiene)
Check from the hand where the IV line is going on
Check from the limb in which no dextrose infusion going on
Care of finger prick site after checking glucose
|
Done – Y£ N£
Done – Y£ N£
Checked- Y£ N£
Checked– Y£ N£
Checked– Y£ N£
Y£ N£
Comments, if any
Checked– Y£ N£
Checked– Y£ N£
|
|
Log (Appendix 3)
|
|
|
Recording and analysis:
On paper
|
Y£ N£
|
Cont'd
Cont'd
|
Table 4. Checklist for Ward Patients
|
|
Ward ID
|
Audit No. 1
|
Audit No. 2 onwards
|
|
E-enabled (Integrated personalized diabetes management [IPDM])
Escalation matrix in place
|
Y£ N£
Y£ N£
|
|
|
Action:
Change in diet
Change in frequency and timing of glucose testing
Change in OAD/ insulin type.
Change in insulin dose
Use of dextrose or any other IV fluids
|
Done – Y£ N£ If Y, Specify
Done – Y£ N£ If Y, Specify
Done – Y£ N£ If Y, Which OAD?
Done – Y£ N£ If Y Specify
|
|
Storage (e.g., strips)
Disposal: hospital
Plastics
Sharps
Blood-stained swabs
|
Storage Done as per instruction Y£ N£
Done – Y£ N£
Done – Y£ N£
Done – Y£ N£
|
|
Appendix 3: Glucose Monitoring and Insulin and/or OAD Log (Ward patients)
|
|
Day/ Time
|
|
Fasting
|
AB
|
BL
|
AL
|
BD
|
AD
|
3 am
|
Comments (eg any change in diet, physical activity, illness, antibiotics)
|
|
Date
|
Blood Glucose
|
|
|
|
|
|
|
|
|
|
Insulin Dose
|
|
|
|
|
|
|
|
|
|
Date
|
Blood Glucose
|
|
|
|
|
|
|
|
|
|
Insulin Dose
|
|
|
|
|
|
|
|
|
|
Date
|
Blood Glucose
|
|
|
|
|
|
|
|
|
|
Insulin Dose
|
|
|
|
|
|
|
|
|
|
Date
|
Blood Glucose
|
|
|
|
|
|
|
|
|
|
Insulin Dose
|
|
|
|
|
|
|
|
|
|
Date
|
Blood Glucose
|
|
|
|
|
|
|
|
|
|
Insulin Dose
|
|
|
|
|
|
|
|
|
|
Date
|
Blood Glucose
|
|
|
|
|
|
|
|
|
|
Insulin Dose
|
|
|
|
|
|
|
|
|
|
Date
|
Blood Glucose
|
|
|
|
|
|
|
|
|
|
Insulin Dose
|
|
|
|
|
|
|
|
|
BB, BL, BD: Before Breakfast, Lunch, Dinner
AB, AL, AD: After Breakfast, Lunch, Dinner
|
Table 5. Checklist for Emergency/Casualty Patients
|
|
| |
Audit 1
|
Audit 2
|
|
|
Procurement:
Type of glucometer- glucose oxidase or glucose dehydrogenase (which brand)
of, ancillaries i.e., lancets, strips, (which brand)
|
Procurement
Recommended brand of glucometer
Recommended brand of ancillaries
Comments, if any
|
CMEs and CNEs should be conducted regularly (monthly or quarterly). This should be accompanied/followed by audits at frequent intervals.
These audits are targeted at emergency nurses. It is expected that they will follow good glucometric practices.
They should be able to refer the patient as well as their caregivers to the right health care provider upon discharge.
|
|
|
Usage pattern:
Individual bed
Shared beds
|
Individual£
Shared£
|
|
|
Pre-testing practices
Glucometer battery
Sanitization
a) Fingertip sanitization
b) Glucometer disinfection
Check from the hand where the IV line is going on
Check from the limb in which no dextrose infusion going on
|
Glucometer
battery working – Y£ N£
Sanitizatiion Y£ N£
Done Y£ N£
Done Y£ N£
Needle Checked Y£ N£
Comments, if any
|
|
| |
|
|
Testing:
Glucometer check
Confirm glucose units (mg or mmol)
Procedure of pricking
Care of finger prick site after checking glucose
Rotating site of finger prick
Troubleshooting (poor circulation, lack of hygiene)
|
Done – Y£ N£
Done – Y£ N£
Checked – Y£ N£
Checked – Y£ N£
Checked – Y£ N£
Comments, if any
|
|
|
|
Log (Appendix 4)
|
|
|
|
|
Recording:
E-enabled matrix/hospital information system
|
Done – Y£ N£
|
|
|
|
Action:
Change in insulin dose/insulin type
Last insulin dose and time before discharge
Escalation/De-escalation matrix
|
Done – Y£ N£
If, Y Specify dose & type,
Last insulin dose………..........
time before discharge……….
Specify
|
|
|
|
Storage of strips disposal: hospital
Plastics
Sharps
Blood-stained swabs
|
Storage done as per instruction – Y£ N£.
Done – Y£ N£
Done – Y£ N£
Done – Y£ N£
|
|
|
|
Appendix 4: Frequency of Monitoring and Insulin Log (Emergency/Casualty patients)
|
|
Date: …………………
Type of Insulin……………….
|
|
Day/Time
|
8 am
|
8:15 am
|
8:30 am
|
8:45 am
|
9:45 am
|
11:00 am
|
|
|
GCS
|
|
|
Plasma Glucose
|
|
|
|
|
|
|
|
|
IV Infusion
|
|
|
|
|
|
|
|
|
Oral Intake
|
|
|
|
|
|
|
|
|
Insulin
|
|
|
|
|
|
|
|
Date:
|
Table 6. Checklist for ICU Patients
|
|
Patient - Name
|
Audit 1
|
Audit 2
|
|
Procurement:
Type of glucometer- glucose oxidase or glucose dehydrogenase (which brand)
of ancillaries, i.e., lancets, strips, (which brand)
|
Procurement
Recommended brand of glucometer
Recommended brand of ancillaries
Comments, if any
|
CMEs and CNEs should be conducted regularly (monthly or quarterly). This should be accompanied/followed by audits at frequent intervals.
This audit is targeted at ICU nurses. It is expected that they will follow good glucometric practices. They should be able to refer the patient as well as their caregivers to the right healthcare provider upon discharge.
|
|
Usage pattern of glucometer: (tick any)
Individual or
Shared
|
Individual£
Shared£
|
|
Pre-testing practices:
Glucometer battery (check after how much time)
Sanitization;
a) Fingertip sanitization
b) Glucometer disinfection
Check from the hand where the IV line is going on
Check from the limb in which no dextrose infusion going on
|
Glucometer
battery working – Y£ N£
Sanitization
Done Y£ N£
Done Y£ N£
Checked Y£ N£
Comments, if any
|
|
Testing:
Glucometer check
Confirm glucose units (mg or mmol)
|
Done – Y£ N£
Done – Y£ N£
|
|
Procedure of pricking
Care of finger prick site after checking glucose
Rotating site of finger prick
Troubleshooting (poor circulation, lack of hygiene)
|
Checked – Y£ N£
Checked – Y£ N£
Y£ N£
|
| |
Comments if any
|
|
Log (Appendix 5)
|
|
|
Recording:
E-enabled system (Hospital information system)
On paper (Structured Reports)
|
Done – Y£ N£
Done – Y£ N£
|
Cont'd
Cont'd
|
Table 6. Checklist for ICU Patients
|
|
Patient - Name
|
Audit 1
|
Audit 2
|
|
Action:
Change in insulin dose/type
Escalation/De-escalation rules
|
Done – Y£ N£
If Y Specify dose & type
Y£ N£
Comments, if any
|
|
|
Storage (e.g., of strips) Disposal: hospital
Plastics
Sharps
Blood-stained swabs
|
Storage Done as per instruction Y£ N£
Done – Y£ N£
Done – Y£ N£
Done – Y£ N£
|
|
Appendix 5: Frequency of Monitoring and Insulin and/or OAD Log (ICU Patients)
|
|
Day/Time
|
Fasting
|
2 hours after breakfast
|
BL
|
2 hours after lunch
|
BD
|
2 hours after dinner
|
3 am
|
Random
|
| |
|
BG/Insulin rate
|
|
|
|
|
Time
|
Glucose value
|
|
Monday
|
|
|
|
|
|
|
|
|
|
Tuesday
|
|
|
|
|
|
|
|
|
|
Wednesday
|
|
|
|
|
|
|
|
|
Week:….Date Onwards
|
Day/Time
|
8 am
|
10 am
|
Noon
|
2 pm
|
4 pm
|
6 pm
|
8 pm
|
Random
|
| |
|
BG/Insulin rate
|
|
|
|
|
|
|
|
Monday
|
|
|
|
|
|
|
|
|
|
Tuesday
|
|
|
|
|
|
|
|
|
|
Wednesday
|
|
|
|
|
|
|
|
|
Week:….Date Onwards
presenting to the health care systems at different levels of point-of-care. Tables 3-6 and Appendices 1-5 describe the checklist and logs for outpatients, ward patients, Emergency/Casualty and ICU patients.
Conclusion
Glucometric guardianship aims to ensure optimal glycemic management. It is a process of allowing appropriate assessment, monitoring, and analysis of glucose levels regularly. The aim of glucometric guardianship is to (i) enable alertness in glycemic management; (ii) agility in anticipating and detecting suboptimal glycemic parameters, and (iii) response to glycemic variability. These checklists will enable health care providers to enhance glycemic management, anticipate and identify suboptimal glycemic parameters, and respond effectively to glycemic variability.
Acknowledgment
The authors would like to appreciate the valuable contribution of Shubhda Bhanot, Dr Sruti Chandrasekaran,
Dr Saptarshi Bhattacharya, Dr Gaurav Beswal, Dr Rahul Baxi, Dr Nilakshi Deka, Dr Mihir B Shah, Dr Sneha Kothari,
Dr Vaishali Deshmukh, Dr Snehal R Tanna, Dr Sunder Krishnan, Dr Mohan Badgandi, Dr Ganapathi Bantwal, Dr Priya Chinnappa, Dr Rajagopalan Sundararaman, Dr Adlyne R Solomon, Dr KD Modi, Dr Ravi Sankar Erukulapati, Dr Arun Mukka, Dr Binayak Sinha, Dr Sunil Mishra, Dr Ganesh Sudhakar Rao Jevalikar, Dr Abhay Inderjit Ahluwalia, Dr Vineet K Surana, Dr Chandar Mohan Batra, Dr Tarunika Bawa, Dr Anupam Biswas in the development of the checklists.
References
- Weinstock RS, Aleppo G, Bailey TS, Bergenstal RM, Fisher WA, Greenwood DA, et al. The role of blood glucose monitoring in diabetes management. Arlington (VA): American Diabetes Association; 2020 Oct.
- Zafar S, Yaddanapudi SS. Parkinson Disease. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470193/
- ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al; American Diabetes Association. 1. Improving care and promoting health in populations: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S10-S18.
- Anderson JE, Gavin JR, Kruger DF. Current eligibility requirements for CGM coverage are harmful, costly, and unjustified. Diabetes Technol Ther. 2020;22(3):169-73.
- Miller EM. Using continuous glucose monitoring in clinical practice. Clin Diabetes. 2020;38(5):429-38.
- Kalra S, Priya G, Gupta Y. Recent advances in endocrinology. JPMA. 2018;68:963-965.
- Kalra S, Verma SK, Bhattacharya S. Glycemic guardianship: World Health Organization leads the way. Asian J Diabetol. 2022;23(4):5-6.
- Kalra S, Mittal S. COVID-19 and diabetes: Covidiabetology. J Pak Med Assoc. 2020;70(6):954-5.
- Kalra S, Agrawal N, Kapoor N, Kalhan A, Teelucksingh J, Sahay R. Glucometric guardianship. Indian J Clin Pract. 2023;33(8):31-2.
- Goldberg PA, Bozzo JE, Thomas PG, Mesmer MM, Sakharova OV, Radford MJ, et al. "Glucometrics"—assessing the quality of inpatient glucose management. Diabetes Technol Ther. 2006;8(5):560-9.
- Thomas P, E Inzucchi S. An internet service supporting the quality assessment of inpatient glycemic control.
J Diabetes Sci Technol. 2008;2(3):402-8.
- Saulnier GE, Castro JC, Cook CB. Impact of measurement error on predicting population-based inpatient glucose control. Future Sci OA. 2019;5(5):FSO388.
- Maynard G, Schnipper JL, Messler J, Ramos P, Kulasa K, Nolan A, et al. Design and implementation of a web-based reporting and benchmarking center for inpatient glucometrics. J Diabetes Sci Technol. 2014;8(4):630-40.