Abstract
Osmotic demyelination syndrome (ODS), a disease affecting chronic alcoholic and malnourished patients was described by Adams and colleagues in 1959. It is also known as pontine myelinolysis. Pontine myelinolysis can be subdivided into central pontine myelinolysis (CPM) and extrapontine myelinolysis (EPM) depending upon the level of demyelination, within the pons or outside the pons, respectively. Rapid correction of hyponatremia contributes to the pathogenesis of ODS. Whenever a chronic alcoholic and/or malnourished develops confusion, quadriplegia, pseudobulbar palsy and pseudocoma (Locked-in-syndrome) over a period of several days, a high index of suspicion for ODS must be held.
Keywords: Osmotic demyelination syndrome, central pontine myelinolysis, extrapontine myelinolysis, hyponatremia
Introduction
Osmotic demyelination syndrome (ODS), was described by Adams et al in 1959 as a disease affecting alcoholics and malnourished people. The etiology of ODS was not known for a long time but few authors suspected the cause to be either toxin or nutritional deficiency. ‘Central pontine’ indicates the site of lesion and the term ‘myelinolysis’ was used to emphasize that myelin was affected preferentially compared to other neuronal elements. Central pontine myelinolysis (CPM) is a noninflammatory, demyelinating condition characterized primarily by the systemic, noninflammatory destruction of myelin sheath in the basis pontis and primarily results from aggressive correction of hyponatremia.
In 1983, Laureno et al suggested rapid correction of hyponatremia as the cause for the condition, based on experimental data on animal model. They suggested that the condition could be prevented by correcting hyponatremia by <10 mmol/L in 24 hours.
Although uncommon, ODS has been reported at a rate of 0.4-0.56% for patients admitted to neurology services and 0.05% of cases admitted in a general hospital.
A study found 0.3-1.1% of patients with unsuspected CPM during autopsies, with a greater percentage of CPM noted in patients with liver transplant and chronic liver disease. An autopsy-based study documented a prevalence rate of 0.25-0.5% in the general population and 10% in patients undergoing liver transplantation.
Case Report
A 63-year-old male presented to the emergency department (ED) with an unsteady gait, giddiness and left-sided weakness. His medical history was significant for hypertension, on irregular treatment and history of
consumption of alcohol in the past. He had retrospective history of intravenous (IV) fluid infusion at a private hospital 2 hours prior to the initial presentation to ED.
General physical examination was unremarkable. Neurological examination revealed that the patient was alert, oriented with facial deviation towards right side and power was grade 0/5 both in left upper limb (UL) and lower limb (LL) with NIHSS score of 11. Fundus examination was normal. An initial diagnosis of cerebrovascular accident (CVA) left hemiplegia with left upper motor neuron (UMN) facial palsy was made.
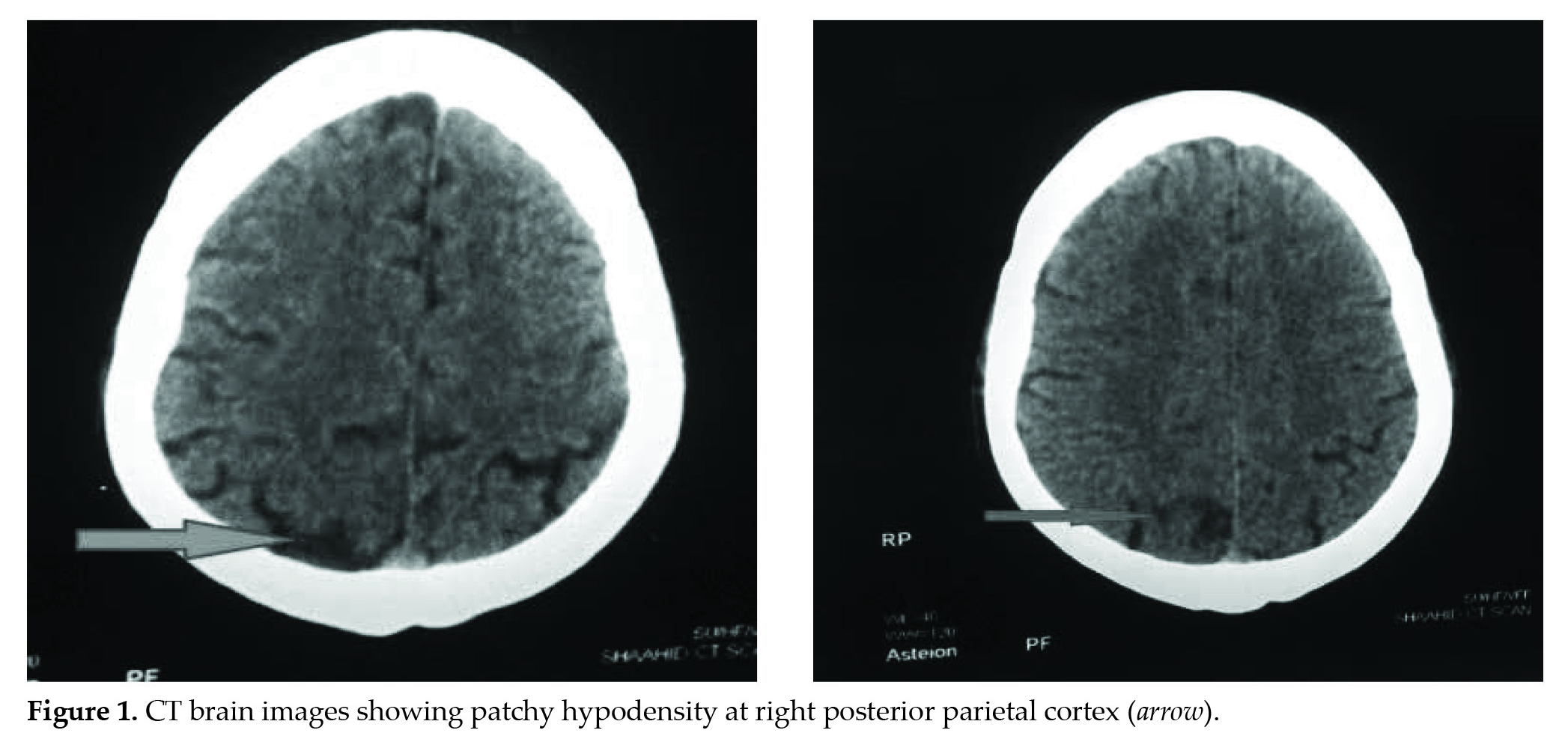
Laboratory investigation showed that the patient had significant hypernatremia (149 mmol/L) at the time of presentation. Computed tomography (CT) of the brain (Fig. 1) performed approximately 3 hours after initial presentation was consistent with features of CVA (right parasagittal posterior parietal cortex).
On next day, 16 hours after initial presentation he developed dysarthria, dysphagia and inability to use his right LL. Motor examination showed decreased tone in left UL and LL, power of grade 0/5 in left UL and LL; grade 2/5 in right LL with exaggerated deep tendon reflexes, left equivocal plantar response (NIHSS score of 16). Pupils were pinpointed and sluggishly reacting to light. Ocular fundus examination was normal. Patient was shifted to intensive care unit (ICU) and mechanically ventilated. Provisional diagnosis of probable posterior circulation restroke at the level of pons was made. Laboratory investigations revealed normal cell count and renal parameters. However, repeat sodium level was elevated (146 mmol/L).
Magnetic resonance (MR) (Fig. 2) imaging, performed 36 hours after the initial CT, showed well-defined area of diffusion restriction in the lower central pons bilaterally.
Differential diagnosis of pontine infarct, pontine hemorrhage and ODS was made.
In view of IV fluid infusion prior to presentation to our ED and clinical features of ataxia, quadriparesis, dysphagia, dysarthria without ophthalmoplegia and sensory loss suggestive of ODS with two elevated values of sodium and also classical MRI findings of diffusion restriction in the lower central pons bilaterally, diagnosis of ODS was made and treated accordingly. During the course of hospitalization, patient developed VAP for which he was treated and was discharged 3 weeks after admission with residual minimal left hemiparesis.
Discussion
It is important to differentiate between structural and metabolic causes of neurological deficits, and if structural, the level of lesion has to be localized. Lower cranial nerve palsies and bilateral findings point towards lower pontine lesion, the cause of which may be:
- Pontine infarct
- Pontine hemorrhage
- Osmotic demyelination syndrome (ODS).
Pontine Infarct
Isolated pontine strokes are relatively frequent, but they can occur as part of the posterior circulation infarction. Ventral infarcts are the most common type of isolated pontine infarction (51-58%).
Anteromedial infarct causes hemiparesis or hemiplegia, contralateral ataxia, dysarthria, dysphagia, nystagmus and often ipsilateral facial palsy. Less frequently associated is contralateral loss of proprioception, paresis of the ipsilateral horizontal gaze and internuclear ophthalmoplegia. Anterolateral infarct may produce hemiparesis, ataxia, loss of position sense and loss of vibration sense. Pure motor stroke, ataxic hemiparesis, dysarthria-clumsy hand or sensorimotor stroke are the other forms of manifestations of the anterolateral strokes.
Dorsolateral pontine strokes may lead to contralateral hemiparesis, ipsilateral facial weakness, ipsilateral loss of facial pain and temperature sensation, hearing loss and ataxia. Rostral dorsolateral pontine infarct can manifest as ipsilateral Horner’s syndrome, contralateral ataxia and contralateral loss of body pain and temperature sensation.
Pontine Hemorrhage
Classic clinical presentation of pontine hemorrhage is acute onset of coma, tetraparesis, respiratory failure and oculomotor signs, and most patients have diminished sensorium. Prodromal symptoms, such as headache, nausea and vomiting, respiratory dysfunction and dysarthria may be present.
Osmotic Demyelination Syndrome
ODS is characterized by its subacute sequential presentation, initial encephalopathy or seizures, followed by rapid recovery in relation to electrolyte or osmolality correction, and subsequent clinical deterioration. Clinical manifestations include predominant ataxia (reflecting involvement of pontocerebeller fibers), dysarthria, dysphagia, quadriparesis and alteration in sensorium. Pupillary and oculomotor signs were less frequently noted. Extrapontine extension results in behavioral abnormalities and movement disorders. The transverse pontocerebellar fibers are most frequently involved, followed by rostrocaudal tracts. Tegmentum and corticospinal tracts are usually spared.
Conclusion
Presence of seizures, predominant ataxia, quadriparesis, pupillary and oculomotor signs with hypernatremia and classical MRI findings of bilateral diffusion restriction are noted in ODS. Behavioral and abnormal movements occur if there is an extrapontine extension. Absence of Horner’s syndrome, internuclear ophthalmoplegia and sparing of primary and posterior column sensation favors ODS.
Early diagnosis and early differentiation between structural and metabolic cause of neurological deficits will help avoid inadvertent usage of anticoagulants, antiedema measures, repeated imaging (radiation exposure) and stroke resuscitative interventions. Targeted therapy towards the correction of metabolic parameters will lead to a favorable outcome.
Suggested Reading
- Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry. 1959;81(2):154-72.
- Laureno R. Central pontine myelinolysis following rapid correction of hyponatremia. Ann Neurol. 1983;13(3):232-42.
- de Souza A, Desai PK. More often striatal myelinolysis than pontine? A consecutive series of patients with osmotic demyelination syndrome. Neurol Res. 2012;34(3):262-71.
- Kallakatta RN, Radhakrishnan A, Fayaz RK, Unnikrishnan JP, Kesavadas C, Sarma SP. Clinical and functional outcome and factors predicting prognosis in osmotic demyelination syndrome (central pontine and/ or extrapontine myelinolysis) in 25 patients. J Neurol Neurosurg Psychiatry. 2011;82(3):326-31.
- Bhoi KK, Pandit A, Guha G, Barma P, Misra AK, Garai PK, et al. Reversible parkinsonism in central pontine and extrapontine myelinolysis: A report of five cases from India and review of the literature. Neurol Asia. 2007;12:101-9.
- Newell KL, Kleinschmidt-DeMasters BK. Central pontine myelinolysis at autopsy; a twelve year retrospective analysis. J Neurol Sci. 1996;142(1-2):134-9.
- Kleinschmidt-Demasters BK, Rojiani AM, Filley CM. Central and extrapontine myelinolysis: then...and now. J Neuropathol Exp Neurol. 2006;65(1):1-11.